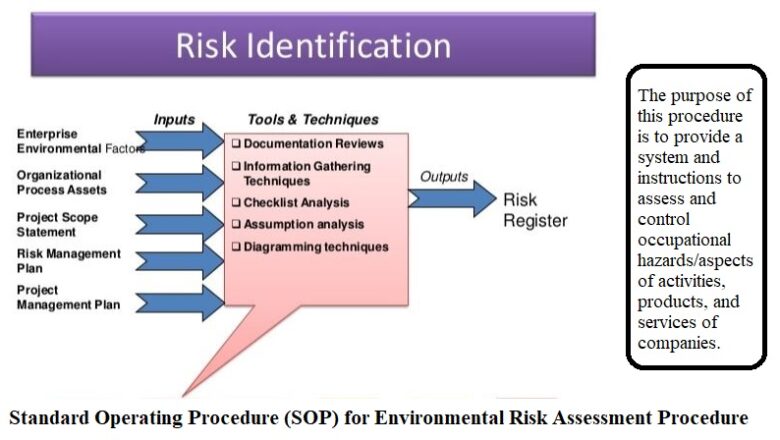
Standard operating procedure for Equipment Qualification
Standard operating procedure for Equipment Qualification is a critical process in various industries, including pharmaceuticals, biotechnology, healthcare, and manufacturing. It ensures that equipment meets specified requirements and is suitable for its intended purpose. Standard Operating Procedures (SOPs) for equipment qualification help maintain consistency, reliability, and compliance with regulatory standards.
Objective:
Objective of this SOP is to address the guidelines for the execution of the Equipment Qualification i.e. (Installation Qualification (IQ)), (Operational Qualification (OQ)) and (Performance Qualification (PQ)). Clearly state the purpose of the SOP, which is to define the systematic process for qualifying equipment to ensure its reliability, accuracy, and compliance ...