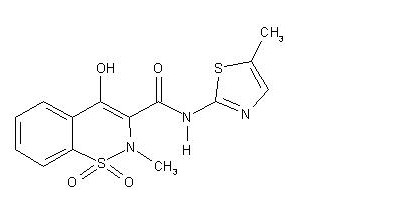
Product Processes and Quality of In Process Material
The purpose of a Product Processes and Quality of In Process Material system is to provide staff and management with objective insight into the process and related work products. Your process and product quality approach supports the delivery of high-quality products by providing project staff and managers at all levels with appropriate visibility into the process and related work products throughout the life of the pharmaceuticals.
Product Processes and Quality of In Process Material
Objective:-
To ensure compliance of Product Processes and quality of in process material.
Responsibility:-
Section Supervisor
Production Manager
QA Incharge
Procedure:-
Issuance of Raw Material & Packaging Components
Check the product name, strength, printing quality and quantity o...