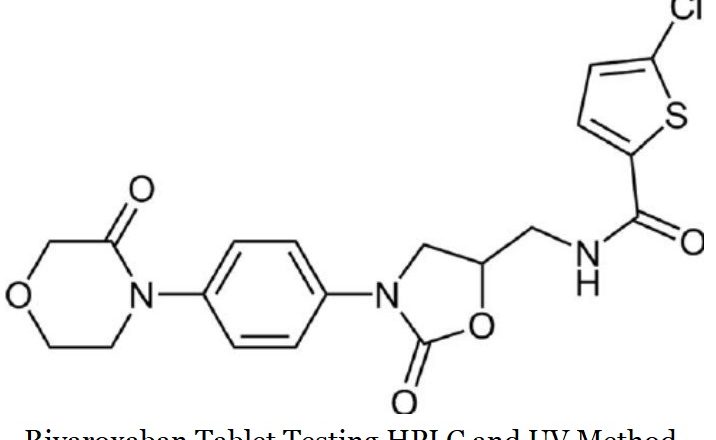
Hplc and UV Testing Method of Montelukast Sodium in Tablet
It is important to note that the specifics of the assay method, including the instrumentation, chromatographic conditions (for HPLC), wavelength selection (for UV spectrophotometry), and sample preparation steps, may vary depending on the official compendial method, published literature, or specific requirements of your laboratory. Hplc and UV Testing Method of Montelukast Sodium in Tablet It is recommended to consult relevant references or validated methods for detailed experimental conditions.
PURPOSE:
To describe the procedure for analysis at the in-process and finished stage of Montelukast Sodium Tablet.
SCOPE:
This SAP gives a detailed outline for the finished product analysis of the Montelukast Sodium Tablet and will cover In process testing activities on physical, chemical &...