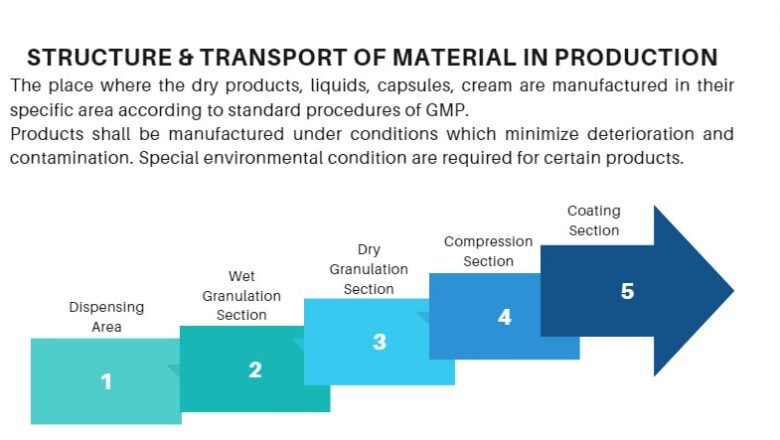
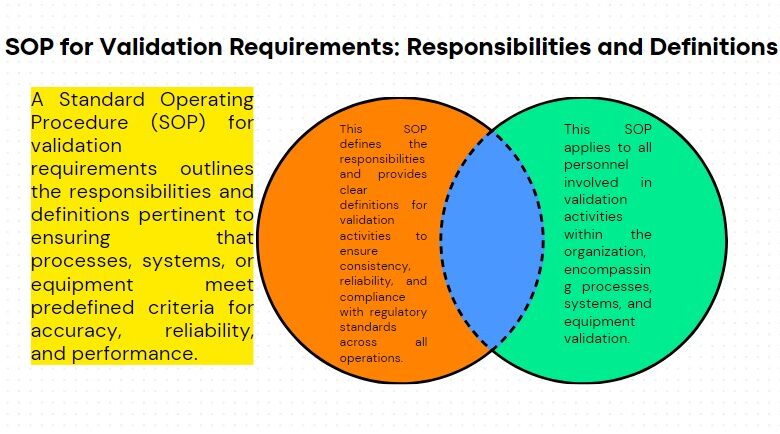
SOP for Accident or Incident Handling
Standard Operating Procedure SOP for Accident or Incident Handling is crucial for ensuring a systematic and effective response.
This procedure applies to all employees, contractors, and visitors. It covers the steps to be taken in the event of an accident or incident involving injury, property damage, or environmental impact.
PURPOSE
To provide a guideline for the handling and investigation procedure to be followed for Dangerous occurrences, accidents, and near misses accident.
SCOPE
Applicable to all dangerous occurrences, accidents, and near-miss accidents.
RESPONSIBILITIES
All departments
DEFINITION
Dangerous Occurrence:
It is unplanned, unexpected event which causes or results in the situation in the factory premises, required to be immediately controlled otherwise result...