In the pharmaceutical industry, Defect Classification Strategies in Pharmaceuticals can be classified into various categories based on their nature, severity, and impact on product quality and safety.
Objective:
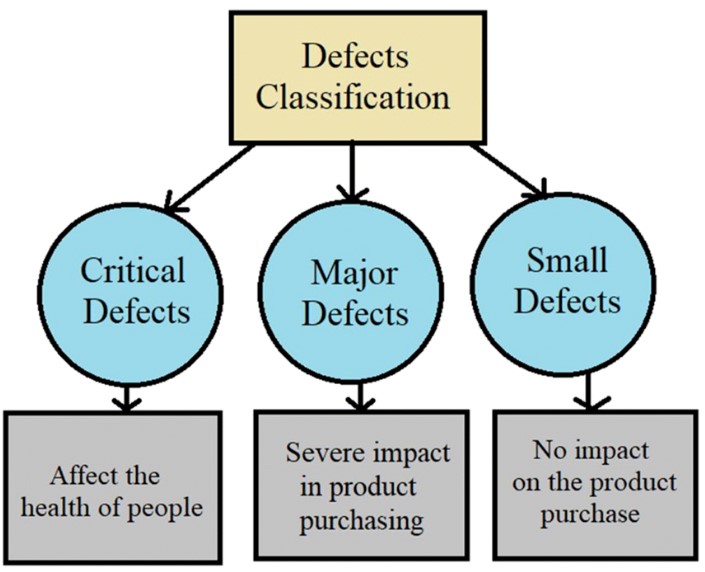
This procedure Defect Classification Strategies in Pharmaceuticals defines the Acceptable Quality Level (A.Q.L) for tablets, capsules, and liquid products.
Scope:
This work instruction applies to all batches of tablets, capsules and liquid products, manufactured and packed at ____________Company plant.
Responsibility:
It is the responsibility of QAI (Quality Assurance Inspector) to follow this procedure.
It is the responsibility of the Production Manager and QA Manager to implement this procedure.
Procedure:
Categories and Limits of A.Q.L:
The batch is acceptable if it complies the following criteria.
Category A.Q.L
- Critical 0.00%
- Major 1.00%
- Minor 2.50%
Samples to be pulled by QAI, from across the batch, as per following sampling plans:
– Table I (sample size code letters).
Note: Follow ‘General Inspection Level II’
– Table II A (Single sampling plans for normal inspection).
– Table II B (Single sampling plans for tightened inspection).
Samples to be logged into following documents:
- For Tablets:
- For Capsules:
- For Liquids:
The QAI must mention the number of samples required as per sampling plan into above relevant documents and perform the AQL using the checklist, mention in table II, III, & IV.
A.Q.L Assessment of Tablets:
Place the sample in an appropriate Petri plate and view it for at least 30 seconds under an artificial light using a white background, at a distance of 15-18 inches from the eye.
Take out any defective tablet and categories it according to the definitions, classified in table II.
Place the cover plate on the Petri plate and lift off the plate. View the reverse side of the tablets as described.
If the does not meet the criteria, mention in step—— then sorting of the whole lot is required.
Defect Classification Strategies in Pharmaceuticals
Table II
Definitions and categorization of the Tablets defects.
Compressed Tablets:
A. Critical Defects:
- Foreign Tablet
- Incorrect Color of Tablet
- Incorrect Engraving on Tablet
B. Major Defects:
- Critical cracks
- Broken Tablets
- Capped Tablets
- Bad shaped Tablets
- Illegible engraving (> 5%)
- Engraving missing
- Picking/Sticking
- Embedded particles
- Black specks (> 0.25 mm)
- Color variation
C. Minor Defects:
- Slight surface blemish
- Engraved defects but still legible
- Chipped tablets < 5%
- Small black specks < 0.2%
II Coated Tablets:
A. Critical Defects:
- Foreign Tablet
- Incorrect Color of Tablet
- Embossing Unreadable
B. Major Defects:
- Embossing readable with ambiguity
- Broken Tablets (Still coated)
- Chipped Tablets > 10% missing
- Bad shaped Tablets
- Extraneous matter adhering / embedded
- Tablets not fully coated i.e. core exposed
- Embedded particles, pimples > 0.3 mm
- Mottled coating
- Orange peel effect (surface similar to orange or lemon)
- Surface coating / matt finish
- Peeling of coating
- Picking or sticking
- Black speck > 0.3mm
- Twining
- Non-uniform distribution of color
C. Minor Defects:
- Coating not free from surface blemish
- Broken chipped tablet between 5-10% missing
- Faded color
- Black specks < 0.25 mm
A.Q.L Assessment of Capsules:
- Place the sample in an appropriate Petri plate and view it for at least 30 seconds under an artificial light using a white background, at a distance of 15-18 inches from the eye.
- Take out any defective capsule and categories it according to the definitions, classified in table III.
- Place the cover plate on the Petri plate and lift off the plate. View the reverse side of the capsules as described in 4.5.1.
- If the does not meet the criteria, which in step 4.1, then sorting of the whole lot is required.
Table III
Definitions and categorization of the Capsules defects. - Two piece DFC (Dry Filled Capsules):
Critical Defects:
- Foreign Capsules
- Incorrect Color of Capsules
- Incorrect printing on Capsules
Major Defects:
- Hole in Capsule
- Cracked or split Capsule
- Powder or oil on exterior of Capsule
- Partial filled Capsule
- Pinholes in Capsules
- Wrinkle on Capsules
- Punches ends
- Short body or cap
- Print interruption Print smeared
- Minor Defects:
- Unprinted Capsules
- Double printed Capsules
- Dented Capsules
- Illegible Printing
- Hangnail Capsules (Piece of cap missing, > 2 mm)
A.Q.L Assessment of Liquids:
- Compare the sample against the description, mentioned in product specification.
- Shake the sample(s) to be examined and transfer about 100 ml of sample into a dry beaker.
- Allow the air bubbles to settle down and examine the sample in front of a viewing cabinet, first against the black back ground for at least 10 seconds then in front of the white background for another ten seconds.
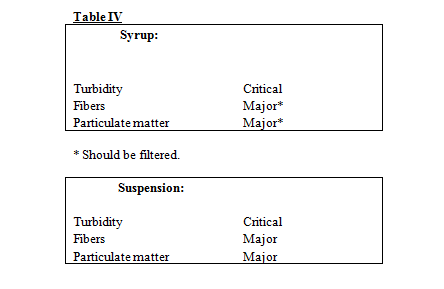
Examine the product for the presence of the following:
- Particulate Matter
- Turbidity
- Fibers
Classify the sample, based on above parameters, according to following table.
Effective classification and management of defects are crucial for ensuring product quality, patient safety, and regulatory compliance in the pharmaceutical industry. Implementing robust quality assurance and quality control processes can help minimize the occurrence of defects and mitigate their impact when they do occur.

