SOP for Identification of Unknown Microorganisms
A Standard Operating Procedure SOP for Identification of Unknown Microorganisms is crucial in a microbiology laboratory to ensure consistency, accuracy, and safety in the process. Keep in mind that specific procedures may vary based on the laboratory's equipment, resources, and protocols.
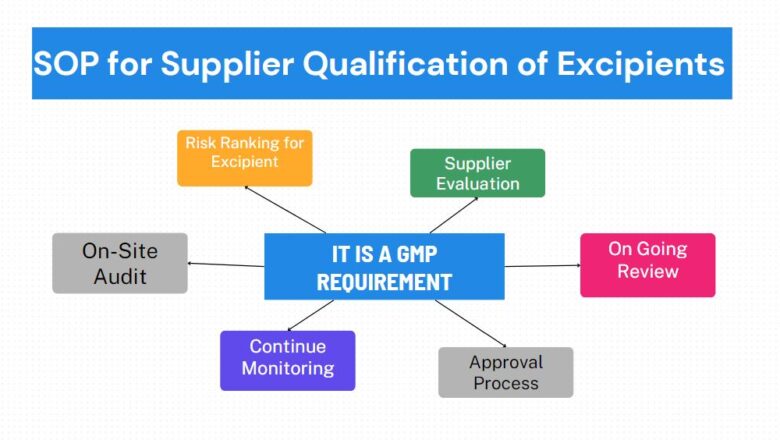
PURPOSE
To describe the procedure for the identification of bacteria as Gram Negative and Gram Positive up to specie level
SCOPE
This procedure is applicable to elucidate the morphology arrangement of bacterial cells.
RESPONSIBILITIES & AUTHORITIES
Manager Quality Control is responsible to ensure that procedure & formats are followed entirely as approved.
Microbiologist is responsible to perform the test.
REFERENCES & REQUIREMENTS
USP
DEFINITIONS & ABBREVIATIONS
Gram Sta...