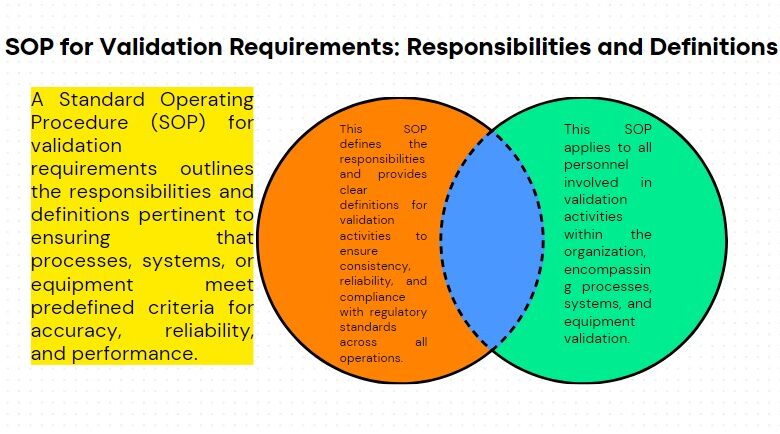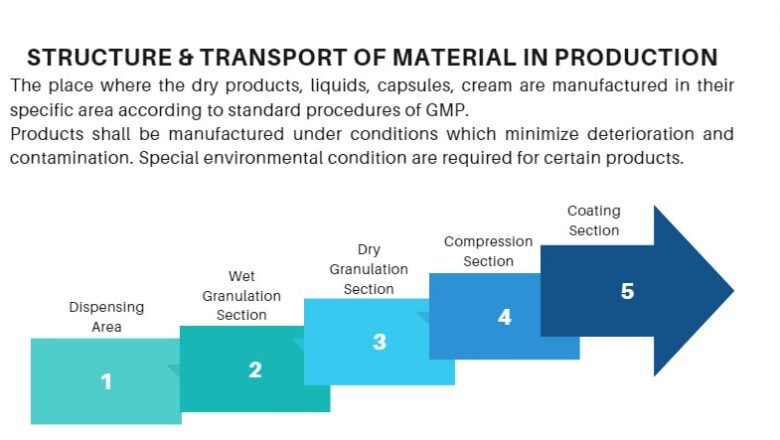
Structure & Transport of Material in Production
Structure & Transport of Material in Production is the place where the dry products, liquids, capsules, cream are manufactured in their specific area according to standard procedures of GMP.
Products shall be manufactured under conditions which minimize deterioration and contamination. Special environmental condition are required for certain products.
Introduction
These conditions should be continuously monitored and corrective action taken, where necessary.
Products are manufactured at temperature 25oC and relative humidity is not more than 50%. Products which are sensitive to humidity are manufactured in special area. Dehumidifier is used for maintaining the humidity less than 50%.
Movement of Materials in the Production Area
According to monthly plan, on the advice of Producti...