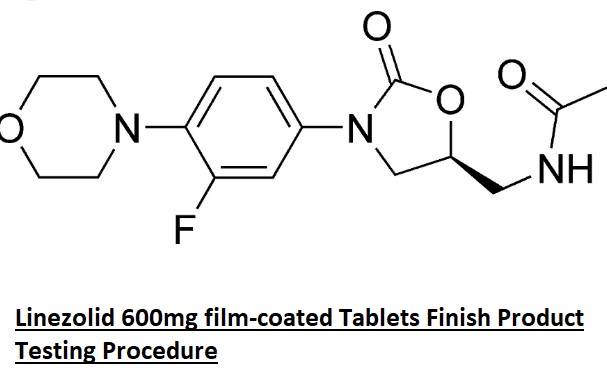
SOP for General Description of Finished Products
SOP for General Description of Finished Products provides a basic overview of the products once they're fully made. It typically includes details like what the product looks like, its size, color, materials used, and any unique features or characteristics. Think of it as a snapshot that helps someone understand what the product is all about without getting into too much technical detail.
INTRODUCTION
A finished product is any pharmaceutical product that has under gone all stages of production, including packing and labeling.
The efficacy and safety of a product can only be assured by analytical monitoring of its quality therefore, the overall purity of a product must therefore, be assessed throughout its manufacturing, storage, distribution and use.
The objective can easily be achi...