SOP for Change Control in Pharmaceuticals
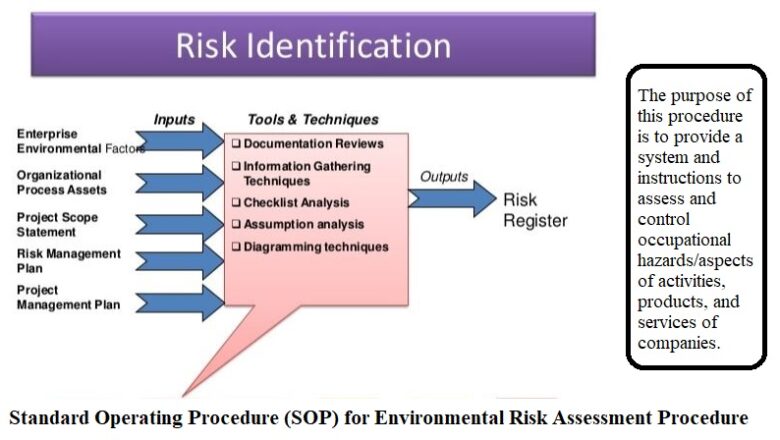
A Standard Operating Procedure SOP for Change Control in Pharmaceuticals industry is a critical document that outlines the systematic approach for managing and documenting changes to processes, systems, equipment, facilities, or any other aspect that may impact product quality, safety, or efficacy. The following is a general outline for creating an SOP for Change Control in Pharmaceuticals:
Purpose
To describe procedure for controlling and documenting changes
Scope SOP for Change Control in Pharmaceuticals
The scope of this document pertains to all major and minor changes related to all approved documents, computerized system, utilities, facilities, equipment, instruments and vendors at www.eggpharma.com
Responsibilities
It is the responsibility of QA to prepare, manage and p...