Structure & Transport of Material in Production is the place where the dry products, liquids, capsules, cream are manufactured in their specific area according to standard procedures of GMP.
Products shall be manufactured under conditions which minimize deterioration and contamination. Special environmental condition are required for certain products.
Introduction
These conditions should be continuously monitored and corrective action taken, where necessary.
Products are manufactured at temperature 25oC and relative humidity is not more than 50%. Products which are sensitive to humidity are manufactured in special area. Dehumidifier is used for maintaining the humidity less than 50%.
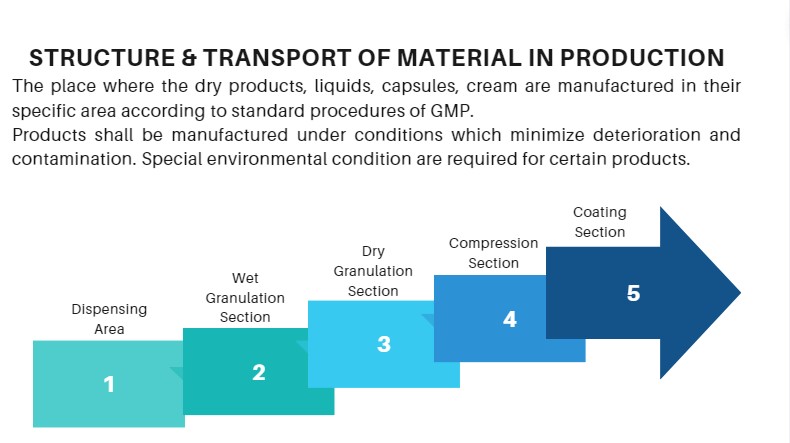
Movement of Materials in the Production Area
According to monthly plan, on the advice of Production Manager, dispensing officer originates the BPR.
Locker
All the raw/packing materials received from the warehouse are first of all placed in the Material Locker. Each container of material is thoroughly cleaned with duster and then transferred to the dispensing section under the supervision of Store incharge and dispensing officer.
Dispensing Area
In this area, raw/packing materials are dispensed on the raw/packing material requisition slip for the manufacturing/packing of the products. Weighing and measuring equipment used in dispensing of raw materials shall be daily calibrated.
The raw materials are weighed in the following types of containers:
- Poly bags
- Plastic bags
- Corrugated drums
Responsibility
- Dispensing process is done under the supervision of Dispensing Officer and Quality Control Inspector.
- Each container is labeled with Raw Material Label which is shown at Appendix-I.
- After complete dispensing of the batch of a product, all the raw materials are placed in the Material Lockers. Each locker is labeled with Raw
- Material Dispensed For which is shown at Appendix-II.
Wet Granulation Section
The raw materials for the manufacturing of a product are dispensed in the Dispensing Section according to the Production Plan. The Production Supervisor checks the weight of all the raw materials according to Raw Material Requisition Slip. Then raw materials for the manufacturing of wet grains are shifted in this section on the BPR Raw Material Requisition Slip. The process of wet granulation is done according to master formula in the Diosna Mixer. Structure & Transport of Material in ProductionBefore starting the process of granulation, Machine Cleaning Certificate is pasted on Diosna Mixer which is shown at Appendix-III. Then all the raw materials are transferred to the Diosna Mixer and mixed, and the solution is added for wet granulation and again mixed for 10 minutes.
Responsibility
Wet granulation process is done under direct supervision of Prod. Supervisor.
Drying Area
After completion of the wet granulation, the wet granules are transferred in the trays which are placed in the trolley. This trolley is placed in the Dryer and the temperature is adjusted according to BPR. Drying Chart is pasted over the Dryer which is shown at Appendix-IV.
Dry Granulation Section
The raw materials for the manufacturing of the products are shifted to this section. In the dry granulation process, all he raw materials are sifted with Sifter which is fixed with sieve number as mentioned in the manufacturing method of the product.
Critical operating parameters for each mixing, blending operation should be done in master formula and method monitored during processing.
Final Mixing
The dried granules, after crushing, are transferred to the Plate Type Mixer
and added all other materials as mentioned in the manufacturing method. Mixed for 30 minutes. Final mixed materials are transferred in the corrugated cartons which are labeled with Product Label as shown at Appendix-V.
Responsibility
Complete process of final mixing is done under the direct supervision of Production Supervisor.
Compression Section
- Final mixed materials are transferred to the Compression Section for the preparation of compressed tablets on Kilian Compression Machine RTS and RUD.
- Accurate calibrated weight measuring balance is used for in-process monitoring of tablet weight. Tablet Compression Control Chart is shown at Appendix-VI.
- Each container in the Compression Section is labeled properly with Product Label to prevent mix-up of materials, granules and cores.
- Both compression machines Kilian RTS, RUD are facilitated with effective dust control system.
Responsibility
Compression machine operator is handling this Section under the direct supervision of Production Supervisor.
Coating Section
After completion of compression process, the whole batch is transferred to the Coating Section. The coating process of every product is done according to the BPR.
Responsibility
Coating Operator is responsible for complete coating process of a batch under the direct supervision of Production Supervisor.
Cream Section
Before starting the manufacturing of the batch, the Production Supervisor comes in the Dispensing Section, collects the BPR and checks weights of all the raw materials according to the Raw Material Requisition Slip of BPR, and transfer them to the Cream Section. Cleaning of every equipment is checked by Production Supervisor and Quality Control Inspector and cleaning certificate is pasted over the equipment.
The manufacturing of the batch is done according to the standard manufacturing method as mentioned in the BPR.
Responsibility
The complete manufacturing process is done under the direct supervision of Production Supervisor.
Syrup Section
Before starting the manufacturing of the batch, the Production Supervisor comes in the Dispensing Section, collects the BPR and checks weights of all the raw materials according to the Raw Material Requisition Slip of BPR, and transfer them to the Syrup Section. Cleaning of every equipment is checked by Production Supervisor and Quality Control Inspector and cleaning certificate is pasted over the Manufacturing Tank.
The manufacturing of the batch is done according to the standard manufacturing method as mentioned in the BPR.
Responsibility
The complete manufacturing process is done under the direct supervision of Production Supervisor.
Structure & Transport of Material in Production
Environment Control
Manufacturing of tablets, syrup, cream is done at room temperature (15oC– 30oC) and Relative humidity is not more than 50%. Production area is controlled regarding temperature and humidity and results are recorded. Temperature and humidity are checked by Q.C. supervisor.
Method:
Control is enforced by means of minimum-maximum Hygro Thermometer.
Interval:
Storage conditions will be checked and recorded once a day.
Reports:
Reports of humidity and temperature control is shown in Appendix VII.
If the measured temperature / humidity values under 15oC, over 30oC / under 45%, over 50%, so the Q.C. Manager is to be informed as well as shown the record.
Q.C. Inspector has to sign each enrollment. Protocols are submitted to Q.C. Manager monthly.
Capsule Section
Dispensing of raw materials for different products of capsules is done in the Dispensing Section according to Production Plan. Production Supervisor of Capsule Section comes in the Dispensing Section, collects the BPR and checks weights of all the raw materials according to the Raw Material Requisition Slip of BPR, and transfer them to the Granulation Section. Cleaning of every equipment is checked by Production Supervisor and Quality Control Inspector and cleaning certificate is pasted over the equipment.
After completion of granulation and final mixing, granules are transferred to the Capsule Section for the filling of capsules.
Responsibility
The complete manufacturing process is done under the direct supervision of Production Supervisor.
Packing Department
After complete manufacturing of tablets, capsules, cream and syrup, the supervisor sends intimation to Q.C. deptt. for getting packing released. The packing procedure of capsules, cream and syrup is described as follows:
Packing of Tablets and Capsules
After getting packing released, the finished products (tablets and capsules) are transferred to the Packing Hall on Transfer Slip in the presence of production supervisor and Packing supervisor by weight. Production supervisor and Packing supervisor get the rough estimate of theoretical number of tablets and capsules by its weight. In the meanwhile, blistering machine is set. Structure & Transport of Material in Production After getting blistering released from Q.C. deptt., blistering is started.
- The blistering machine drops four blisters at a time according to the size of the blister on the conveyer belt.
- The conveyer belt conveys these blisters to our checking team. The number of checkers depends upon the number of blisters per stroke. These checkers thoroughly check the blisters and prepare blister set according to the number of tablets per unit pack. These sets are re-checked by Q.C. Inspectors. The purpose of this strict checking is to avoid rejection due to machine.
- According to the speed of the machine, packers are seated on the belt. The blisters are packed in unit packs and put on the belt of the side containing batch no. MRP, manufacturing and expiry dates.
- At the final packing, two packers are seated, one packer checks the batch number and MRP and collects the unit packs and give them to the second packer.
- Throughout the packing procedure, In-process control sheet and Embossing on blistering sheet are maintained as shown in Appendices –VIII & IX.
- After completing final packing, Q.C.I. stamps outer cartons with his signature. Then the packed material is transferred to F.G. Store on ‘Transfer Slip’.
Packing of Syrup
After complete manufacturing of the syrup, Production supervisor sends the intimation to Q.C. deptt. for getting release for filling of syrup. After complete analysis of the finished product, the Q.C. deptt. issue release for filling. In the case of syrup filling, the following steps are taken:
- First of all, bottles are issued from Packing Material Store on Packing Material Requisition Slip of BPR. These bottles are washed in a rotary washing machine in the presence of pressurized running water. After washing, these bottles are dried and sterilized at 115oC-125oC for one hour.
- Then volume is adjusted (30ml, 60ml and 120ml) according to the specification of ‘ finished products on the Masterfilling machine. When the volume is accurately adjusted by the operator, the Packing supervisor checks the volume and allow for filling.
Training
The staff who is responsible for performance of this operating instruction, is taught and trained demonstrably about the content of this SOP along with CGMP training at least after every three months (Training Chart at Appendix-IV). Training is also provided to new employees.
Storage
Russel Sifters are placed in Granulation Section. These are two in number.
Archiving
Only authorized persons get access to SOP. It is protected from unauthorized person. The contents of SOP are stored in computer.

