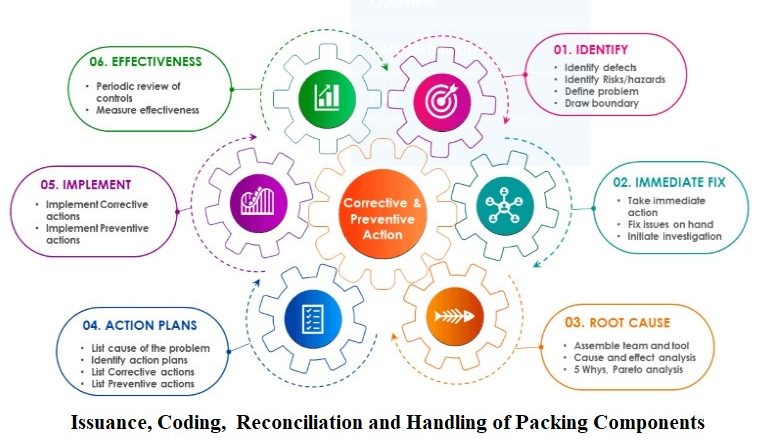
SOP for Metformin Hydrochloride and Sitagliptin Phosphate Tablet Testing by UV and HPLC Method
Sitagliptin + Metformin HCl Tablet is a combination medication used to treat type 2 diabetes. Testing for this medication typically involves a combination of preclinical and clinical studies to evaluate its safety, efficacy, and pharmacokinetics.
Preclinical studies involve testing the medication in animal models to determine its toxicity, pharmacokinetics, and pharmacodynamics. These studies help researchers identify any potential safety concerns and determine appropriate dosages for humans' SOP for Metformin Hydrochloride and Sitagliptin Phosphate Tablet Testing by UV and HPLC Method.
Clinical studies involve testing the medication in human subjects to evaluate its safety and efficacy. These studies are typically conducted in several phases, starting with small studies on unhealthy...