
SOP for General Description of Finished Products provides a basic overview of the products once they’re fully made. It typically includes details like what the product looks like, its size, color, materials used, and any unique features or characteristics. Think of it as a snapshot that helps someone understand what the product is all about without getting into too much technical detail.
INTRODUCTION
A finished product is any pharmaceutical product that has under gone all stages of production, including packing and labeling.
The efficacy and safety of a product can only be assured by analytical monitoring of its quality therefore, the overall purity of a product must therefore, be assessed throughout its manufacturing, storage, distribution and use.
The objective can easily be achieved if the specifications to be applied are based on a validated procedure, which can demonstrate the relationship in quality between the substance under examination.
The quality control department will always depends on the use of valid analytical procedure. It is therefore, important that any analytical procedure for analysis of a product should be systematically evaluated so as to demonstrate that the method is scientifically used under the conditions in which it is applied.
PURPOSE:
The Purpose of this SOP is to be ensuring about the quality of the product how to operate a sample at the stage of manufacturing and at the end of the expiry date and who to validate it.
SAMPLING AT FINISHED PRODUCTS
Summary
Due to great variety of formulations to be analyzed, proper sampling are extremely important for meaningful results. One should ensure that the selected portion of the sample is the true representative of the whole batch. The method to be followed for sampling is as under:
Procedure SOP for General Description of Finished Products
A sampling advice sheet of the product is send by a production supervisor in which analysis of the product is requested.
QUARANTINE
After giving sample advice sheet to quality control department, production supervisor transfer whole batch to quarantine area of finished store.
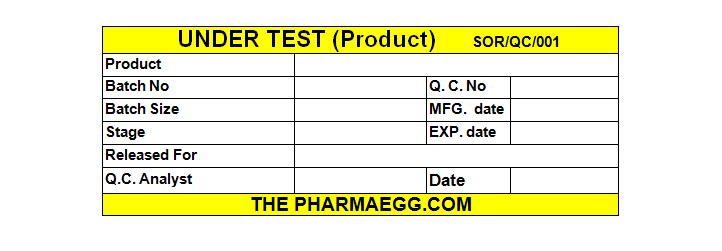
After getting intimation from production supervisor Q.C. Inspector draw sample from at least three cartons and paste under test label on each cartons. The design of under test table is as following:
FIG. 1 UNDER TEST LABEL
Samples of finished product may also be collected during packing. These samples are collected from each running belt after certain period of time
The first three batches of any new drug are usually put under a stability program in order to develop an adequate stability profile. For this stability purpose six additional packs are collected.
TESTING AND RELEASE PROCEDURE
Batches of finished product are subjected to specific test as described in individual SOP’s of each product which must shows that lot samples meet critical specification before these batches are approved for release into channels of distribution.
These tests are usually performed on samples obtained after finishing or during the packing operation. Providing that the batch is not subjected to stress such as heating, compression or gas sterilization etc.
Before analysis at the finished stage each product is given a quality control no. on the following pattern.
Q.C .NO. of each product is given as.
Fyy – 0000
Where
F = Finished product
000 = Serial # (starting from Ist January to 31st December)
yy = Year in which product prepared
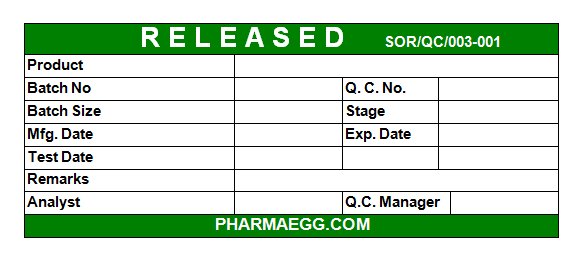
After analysis a release label is pasted on each container or carton when it is going to be market. Released labels is of only one type and have full information in it. The pattern of release label is as under.
FIG. 2 RELEASED LABEL
All products, which are known to be adversely affected by the presence of certain microorganisms, or whose components are highly susceptible to microbial contamination, are tested for microbiological analysis.
Batches that fail to meet critical specifications after laboratory tests are rejected. If these batches warrant it, they are reprocessed to meet critical specifications.
DOCUMENTATION
Separate record of each product is prepared as per specified SOP on registers and also a certificate of analysis of each batch is prepared..
Record of each product is written Batch number wise and with stage wise, with complete tests and calculations.
STORAGE
A product is stored within its specified limits so that it possessed its activity during storage and use i.e. (its shelf life) so that its activity could be maintained as was at the time of manufacturing. The product should be stored under conditions that prevent contamination and as for as possible precaution that should be taken in relation the effects of the atmosphere, moisture, heat and light are indicated in appropriate monographs. Further precautions may be necessary when some products are stored in tropical climate or under other severe conditions.
EXAMPLE
Some products are stored in cold conditions i.e. at 2 – 8°C so the product should be kept at this temperature and maintain it throughout its shelf life. Etc.
STORAGE OF RETENTION SAMPLE:
The retention sample of any finished product should be in sufficient quantity necessary to perform complete quality control analysis twice.
- Finished products should be stored (retained) at appropriate conditions one year after its expiry.
- In process product sample collected at different stages should be retained at appropriate condition for a period until product is released for marketing.
- The first three batches of any new drug are usually kept under a real stability program in order to develop an adequate stability profile.
- If after marketing, a batch under observation found out of stability expectation, an investigation is undertaken the observation.
- Batches of the drug product manufactured immediately before and after the batch in question are usually placed under a stability program.
DISPATCH PROCEDURE
Following steps involved during process for dispatching:
STEP # 1
When a batch is ready for market. A transfer slip is prepared before transferring batch from packing hall to quarantine area of finished goods store. The transfer slip (DFS) has complete information about a batch.
After getting transfer slip, final inspection is done by quality Assurance officer and after getting information of complete analysis a released label is pasted on it and quality control analyst give it to a finished quality control No. Now product is ready for marketing or dispatch to the distributor.
STEP # 2
An Invoice & warranty is prepared as per Drug Act 1976.
TRAINING
PERIODICAL TRAINING:
The staff responsible for performance of this operating instruction, has to be demonstrated periodically about the content of this SOP.
TRAINING FOR NEW EMPLOYEES:
Before starting with the job, the employee has to be demonstrated about the content of this SOP.

