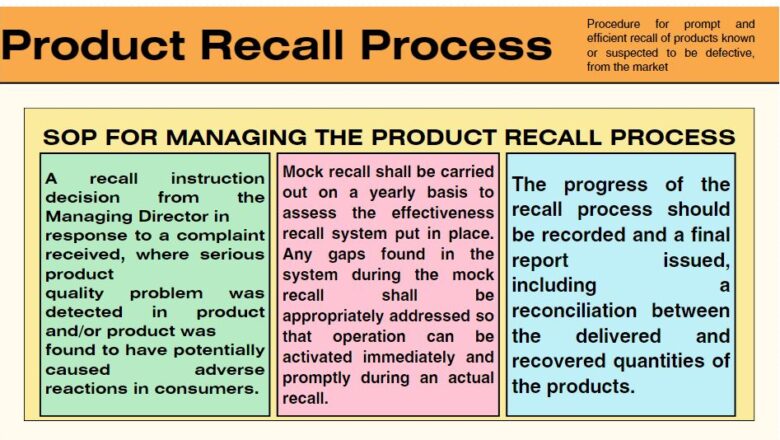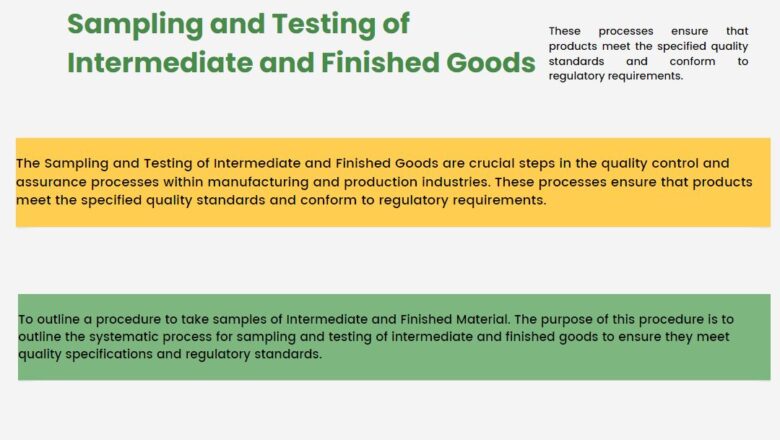
Sampling and Testing of Intermediate and Finished Goods
The Sampling and Testing of Intermediate and Finished Goods are crucial steps in the quality control and assurance processes within manufacturing and production industries. These processes ensure that products meet the specified quality standards and conform to regulatory requirements.
PURPOSE
To outline a procedure to take samples of Intermediate and Finished Material. The purpose of this procedure is to outline the systematic process for sampling and testing of intermediate and finished goods to ensure they meet quality specifications and regulatory standards.
SCOPE:
This SOP applies to sampling of intermediate materials and Finished Materials.
RESPONSIBILITIES:
Production Incharge, QC Incharge and QC Analyst are responsible for representative sampling according to recommended plan...