SOP for Managing the Product Recall Process is crucial for organizations to manage and execute an efficient and effective recall process when necessary. This SOP should provide clear guidelines on how to identify, initiate, and execute a product recall to minimize risks to consumers and comply with regulatory requirements.
OBJECTIVE
The purpose of this Work Instruction is to describe the procedure for the management of the recall, or potential recall, of sales products from the marketplace. Clearly state the purpose of the SOP, which is to establish a systematic process for managing product recalls to ensure the safety of consumers, comply with regulatory requirements, and protect the reputation of the organization.
SCOPE
This Work Instruction applies to all products manufactured and marketed by www.pharmaegg.com. (including physician sample and export sample as well). Define the scope of the SOP, specifying the types of products covered, departments involved, and the stages of the product recall process.
RESPONSIBILITY
Responsibilities are defined in section 4.4 and onwards. Clearly outline the responsibilities of individuals and departments involved in the product recall process, including roles such as the Recall Coordinator, Quality Assurance, Regulatory Affairs, and Communication Team.
PROCEDURE
(DEFINITIONS) SOP for Managing the Product Recall Process
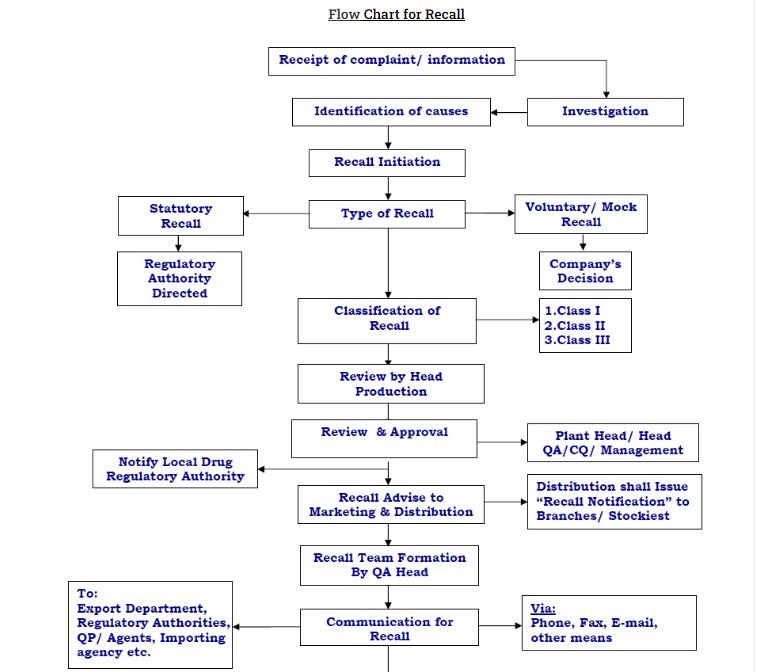
“Recall” is the removal of the entire affected batch of the product from the market; this assumes that the issue was detected while the batch (es) was actually in the market.
Level of Recall
The level of recall is defined in the following way:
- Level I – Stock within Searle Pakistan Limited control in its Warehouses and Depots.
- Level II – Level I plus all known customers (direct account), hospitals, retail pharmacies and wholesalers/distributors but not patients.
- Level III – Level II plus the general public (This will involve the use of the mass media).
Severity of Recall
The severity of recall is defined in the following way:
Medical Director to verify the class of the recalled product which are as follows.
A CRITICAL (as Class 1) Recall is defined as one which is in response to a finding with known or likely to be life-threatening or could cause a serious risk to patients health.
A MAJOR (or Class 2) Recall is one which is in response to a finding which has resulted in actual, or has the potential for, significant adverse drug reaction, (including illness or mistreatment), a serious failure in regulatory compliance or significant adverse business consequences.
A MINOR (or Class 3) Recall is one which is in response to a finding in which there is no potential for significant adverse drug reaction, or is a minor failure in regulatory compliance or which will have minor business consequences.
Examples are given in Appendix – 1
Complaint recipient (Marketing Depts. and IBD”) to forward oral or written complaint to Quality Assurance Manager, according to unit customer complaint procedure No.00000000.
QUALITY ASSURANCE MANAGER will:
- Review the complaint and if the investigation shows that a potential recall situation exists, inform the Director Technical Operations.
- A recall situation will exist if any of the conditions described in Appendix I or the like, is present.
- The Director Technical Operations will inform the Managing Director of the situation.
- The Managing Director will immediately form a recall committee and nominate the coordinator, and will call an
urgent meeting.
RECALL CO-ORDINATOR(S) will:
- Prepare a report detailing findings and recommendations (within 2 hours of the meeting)
- Send the report to the Managing Director for decision.
- The Managing Director will (if necessary) inform the concerned Representatives of Pharma (France) 3M etc., and seek their advice. The communication must be done using most expeditious method.
- *2 The names of representatives of Pharma, 3M etc., and their contact telephone members, in and out of working hours and fax numbers are available in the Managing Director ‘s office.
- In the event of a recall, stock should be recalled within 48 hours if the recall is critical, or 7 working days if the recall is major
or minor. - *3 Director Distribution & Institutional Sales will initiate recall procedure and coordinate with IBL
MEDICAL & REGULATORY AFFAIRS will:
- Inform to the Regulatory Agency (if necessary, depending upon level of recall, and after obtaining permission of the Managing Director), about the recall of product, batch or batches affected, along with reasons for recall.
- DIRECTOR DIS. & INSTITUTIONAL SALES (in co-ordination with National Distribution Manager) will:
- Put all implicated stocks within control under quarantine. (warehouses and Depots throughout the country).
- Obtain details of customers who have been supplied from batches affected.
- Issue recall notifications (by most expeditious method, and subsequently by letters) to all distributors and institutions. They should be asked to collect the batch(es) from retail pharmacies as well.
- For level III recall, inform the general public (this will involve the mass media)
- The text prepared for mass media, should be approved by Managing Director, who will also seek advice of concerned and representative (if necessary) regarding drafting of text.
- If any queries is received from mass media, the Managing Director is the only authority to answer them.
DIRECTOR TECHNICAL OPERATIONS will: (through memo)
- Make a record of action from the commencing of the initiation of recall.
- Liase with Director Materials for the reconciliation of stock.
- If sufficient stock is recovered and there is likelihood that no further stock can be recoverable, then recommend
the closure of recall operations, to the Managing Director.
MANAGING DIRECTOR will:
Inform the Director Operations about the closure of recall operation. (through memo)
DIRECTOR TECHNICAL OPERATIONS will:
- Submit a full report to the Managing Director giving details of reconciliation of stock.
- The Managing Director (if necessary) will inform the concerned representatives, 3M (UK) about the closure of recall operations, detailing all actions taken and reconciliation of stock.
- SOP for Managing the Product Recall Process
- The fate of the recovered stock, will be decided by the Managing Director.
NOTE: All telephone numbers, in and out of working hours, of Searle Pakistan Limited management are avail- able in the plant as well as head office.
MEDICAL & REGULATORY AFFAIRS will:
Report results of the recall to the Regulatory Agency (if necessary)
QUALITY ASSURANCE MANAGER will:
- Retain Recall reports in complaint file for one year beyond the expiry of product.
- The recalled product will then be destroyed in presence of Quality Assurance representative.
- Quality Assurance Manager will retain the recall report and Product Request Form (Stock Destruction), Certificate of Disposal Form in complaint file.
- Contract products manufactured are responsible for the recall of their products.
- SOP for Managing the Product Recall Process
FOR EXPORT PRODUCTS:
The recalled products will be collected as per their distribution record and stored separately in the distributor’s warehouse. The recalled product will be destroyed at distributor’s premises in the presence and Representative.
MOCK RECALL SOP for Managing the Product Recall Process
- The Product Recall Plan must be tested for effectiveness at least once in two years. When mock recall will be carried out.
- Mock recall records must be kept for four years.
- A mock recall will be initiated by QA, who will randomly pick any product and its specific lot number.
- QA will forward the information to Planning & Distribution department of with copy to Materials and Distribution Manager.
- The distribution record will be used to generate a list of customers that have received or potentially received the lot in question.
- The Distribution Department will ensure that the Product Recall List (see attachment) is complied.
- The product Recall List will be reviewed and verified by the Director Distribution & Institution Sales.
- Director Distribution & Institutional Sales will forward the instruction received from QA for further action.
- IBL will inform Director Distribution & Institutional Sales regarding execution of the same procedure.
- Director Distribution & Institutional Sales will inform QA about the action taken.
- After completion of the verification exercise, A mock Recall Report will be released by the QA Manager on the systems’ effectiveness.
- The system will be considered effective if 98% of the batch is correctly accounted for within 03 working days.
In Case the Effectiveness Criteria Are Not Met:
- Distribution department will be required to carry out the an investigation to identify the cause.
- The investigation report must be issued within 05 working days, and is required as a response to the Mock Recall Report.
- The investigation report should include action steps designed to correct the systemic deficiency identified in the report. For each action step, reponsibilities and timings should be defined.
- If there are some outstanding actions after 30 days, a follw-up report will be sent to QA department, giving their Status. A status report will be issued every after 30 days until an action steps have been completed.

