Laboratory Equipment Qualification Procedure SOP outlines the steps and processes for qualifying laboratory equipment to ensure its reliability, accuracy, and compliance with regulatory requirements. Below is a general template for such a procedure. Please note that specific details may need to be tailored based on the type of equipment and regulatory requirements applicable to your laboratory.
PURPOSE
To describe the procedure for defining the responsibilities, planning, protocol writing, performing the qualification activity, and subsequent record generation. The purpose of this Standard Operating Procedure (SOP) is to establish a systematic approach for the qualification of laboratory equipment to ensure accurate and reliable results and compliance with regulatory requirements.
SCOPE
The procedure is applicable to perform qualification activities of the new system and equipment. This SOP applies to all laboratory equipment used for testing, analysis, or other activities within the laboratory.
TERMS & DEFINITIONS
- URS User Requirement Specification
- HOD Head of Department
- MD/CEO Managing Director/Chief Executive Officer
- TS Technical committee Technical Services
- Plant Manager, HOD of the concerned department, GM TS
PROCESS AND RESPONSIBILITY
- Laboratory Manager: Overall responsibility for the equipment qualification program.
- Equipment User: Responsible for daily operation and basic maintenance of the equipment.
- Quality Assurance (QA): Overseeing the qualification process and ensuring compliance.
Technical Committee is responsible to
- Prepare and finalize the list of equipment needed qualification.
- Prepare quarterly schedule for equipment qualification.
- Prepare log sheet for equipment qualification.
- Suggest any new equipment purchase as per the testing need.
HOD (QA/QC) is responsible to approve related documents.
MD/CEO is authorized to ensure the availability of finance.
Relevant department (to which the system/equipment pertains) is responsible for the protocol preparation, conduction, and report preparation of the desired qualification under the supervision of Technical committee HOD (QA/QC) approves (MD/CEO is responsible for authorization of Design Qualification).
AM / Sr. Chemist or Chemist is responsible to ensure the availability of all related information and evidence.
Technical Services Department is responsible to provide any technical assistance for the conduction of Qualification (where needed).
A unique no. is allotted to qualification protocol & report according to the following scheme:
- ID/AA/001P
- ID/AA/001R
- ID is instrument identification
- AA stands for the type of qualification i.e. design qualification.
- 000 serial no. should be same for all qualification of the same equipment
- P is a protocol
- R is a report
Design Qualification (DQ)
DQ is prepared for any new equipment purchased for the first time or in place of the old one (replacement)
Technical committee verified the user requirement or specification and ensures the following related aspects in the protocol.
- Intended use
- User Requirements specification
- Environmental requirements
- Functional /operational specification
- Surety for the conduction of IQ and OQ by the vendor.
- Confirms the internal/external training of lab staff.
- No. of units sold in Pakistan
- Warranty period.
- List of Technical Staff provided by the Supplier.
- a- Total staff
- b- Trained staff for the specific instrument.
- Running cost of the instrument.
- Maintenance cost of the instrument.
- Availability of spares (for preventive maintenance)
- List of previously supplied Equipment to Pharma About.
- Basic Price comparison of the selected supplier/manufacturer
Detail of Instrument/ Equipment selected.
- Mention, if the supplier/ Manufacturer-provided information deviates from the user requirement under deviation report.
- Finally the DQ report is verified by the technical committee, approved by the HOD (Q.A/Q.C), and authorized by MD/CEO.
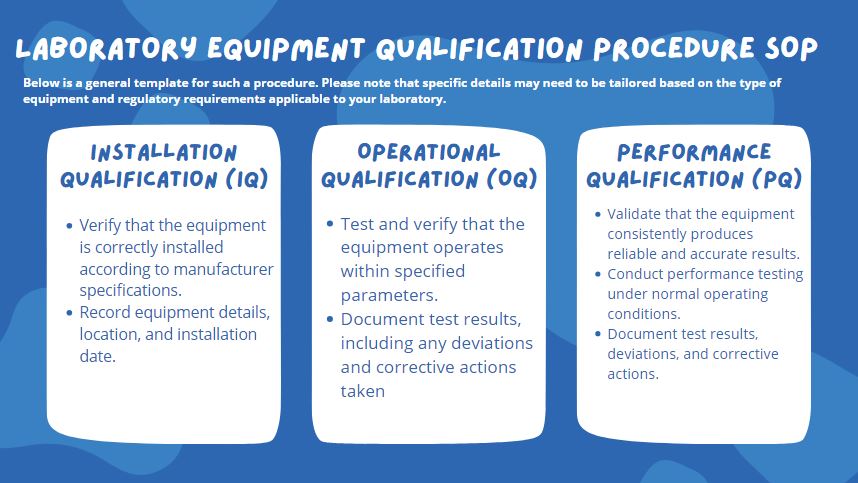
Installation Qualification (IQ)
- New Equipment is installed and verified based on manufacturer Protocol.
- Already installed Equipment need in-house re-installation qualification if
- They are relocated.
- They are serviced from outside.
- Technical committee verifies the installation qualification protocol of the scheduled equipment.
- IQ protocol included the following related aspects.
- Description of the instrument/ equipment to be installed.
- Description of Design, Function, and main components.
- Description of Lubrication system (if any)
- Description of any required supporting utilities (Piping, Connections, Electric, Water /Gas supply, etc.)
- Safety features of Instrument/Equipment.
- Laboratory Equipment Qualification Procedure SOP
Environmental requirements.
a. Critical
b. Non-critical
Checklist of items ordered/Required.
Components
a. Ordered
b. Required
Documents
a. Ordered
b. Required
Main components of Instrument/ Equipment.
- Components
- Specification (Manufacturer’s/ In-house)
- Specification of spare parts (ordered/required).
- Name of spare parts (ordered/required)
- Specification (Ordered/Required).
Product contact surface and material of construction (Optional).
- Item / Part (In product contact)
- Visual Inspection of product contact surface area.
- Material of construction
Recommendation for Installation ( If any).
- Recommendations (Manufacturer/In-house).
- Fill out the report accordingly.
- Report the deviation ( if found ) during installation.
Operational Qualification (OQ)
OQ is performed after IQ.
OQ protocol includes the following related aspects.
Control points and alarms include.
a. Control points / Alarm
b. Acceptance criteria.
c. Results
d. Deviation
Operational Function (Output)
a. Operational Function
b. Acceptance criteria.
c. Results
d. Deviation
List of equipment required for calibration.
a. Apparatus / Instruments
b. Calibration SOP no.
c. Calibration Date.
d. Deviation
Calibration of Equipment/Instrument
a. Calibration SOP no.
b. Calibration parameter.
c. Acceptance criteria.
d. Results
e. Deviation
Specific challenge of the Instrument / Equipment
a. Test (Worst case Situation)
b. Acceptance criteria.
c. Results
d. Deviation
Training Records
a. Training on relevant SOP
b. Trainer name.
c. Trainee name.
d. Signature of Trainer.
e. Deviation
- Fill out the report accordingly.
- Report the deviation (if found) during installation.
Performance Qualification (PQ)
PQ is performed after OQ.
Technical committee prepares performance qualification protocol of the scheduled equipment.
Already performed PQ will be re-performed in-house along with IQ and OQ if
- The equipment is relocated.
- The instrument is serviced from outside.
- Laboratory Equipment Qualification Procedure SOP
List of tests to be performed along with their acceptance criteria.
- Test
- Acceptance criteria.
- Describe in documents
- Document reference no.
- Remarks
- Sampling plan
- Mention about the test to be performed.
- Give the test description.
- Instrument/Equipment/Utility/ Services required for test.
Following parameters will be filled in the protocol.
- Test no.
- Sample no.
- Results
- Acceptance criteria
- Deviation
- Remarks (If any)
- Calculations and Statistical analysis.
- Fill out the report accordingly.
- Report the deviation (if found) during the performance.
RECORD Laboratory Equipment Qualification Procedure SOP
- Quarterly schedule for equipment qualification
- List of equipment selected for qualification study
- Log sheet for equipment qualification
- Design Qualification Protocol prototype
- Design Qualification Report prototype
- Installation Qualification Protocol prototype
- Installation Qualification Report prototype
- Operational Qualification Protocol prototype
- Operational Qualification Report prototype
- Performance Qualification Protocol prototype
- Performance Qualification Report prototype
REFERENCE
USP – 44
DISTRIBUTION
- HOD (QA/QC)
- HOD (TS)
- All Concerned Staff
- Plant Manager
Remember to adapt this template to your specific laboratory, equipment, and regulatory environment. Ensure that your organization’s quality management system and relevant regulations are taken into account when creating and implementing this SOP.


This gateway is incredible. The splendid substance displays the administrator’s commitment. I’m overwhelmed and envision more such astonishing material.
thank you so much