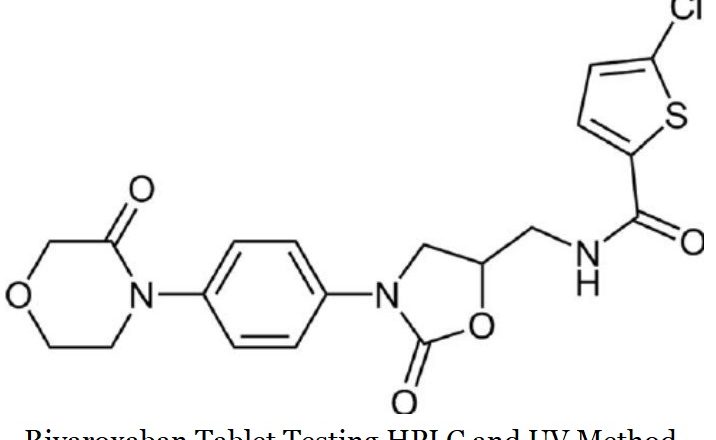
Rivaroxaban Tablet Testing HPLC and UV Method
In terms of testing, the in-process stage of Rivaroxaban tablet testing would typically involve quality control measures to ensure that the tablets are being produced according to the intended specifications. This could include tests to check the tablet's weight, thickness, hardness, and disintegration time. Rivaroxaban Tablet Testing HPLC and UV Method may also undergo testing for purity and content uniformity.
The finished stage of testing involves a series of quality control tests to ensure that the tablets meet the required standards for safety, efficacy, and quality before they are released for distribution. These tests could include identification tests, assay tests, dissolution tests, and impurity tests. The tablets may also undergo stability testing to ensure that they remain ef...










