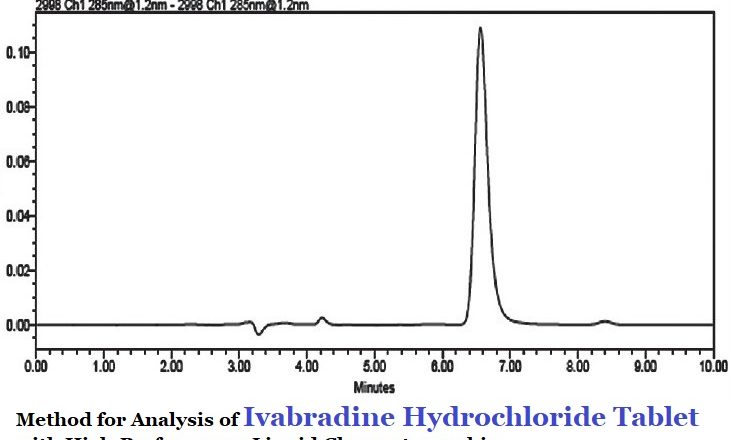
Linezolid 600mg film-coated Tablets Finish Product Testing Procedure
Linezolid 600mg film-coated Tablets Finish Product Testing Procedure, like other pharmaceutical products, is a critical step in ensuring their safety, efficacy, and quality. Below is an overview of the typical testing procedures involved in the production and quality control of Linezolid tablets:
It's essential to note that specific testing procedures may vary depending on the country, regulatory agency, and pharmaceutical company. Manufacturers must adhere to good manufacturing practices (GMP) to maintain the quality and consistency of their products.
The goal of these testing procedures is to ensure that Linezolid 600 mg tablets are safe, effective, and of high quality, providing patients with the expected therapeutic benefits while minimizing risks and maintaining product consiste...