In the post so deeply describe the Handling of Retained Samples & Batch History Records which help Pharmaceuticals in maintaining records.
Objective
To describe the Handling of Retained Samples & Batch History Recordsarticle for receive, review, retention & issuance of Batch History & Retained Sample in compliance of cGMP.
Scope
This procedure is applicable at Pharma.
Responsibilities
Manager QC/QA
Officer Quality Assurance / Quality Management & Quality Control
Inspector Quality Assurance & Lab attendant
Definitions
Batch records
All documents are related to making bulk products or finished products. They provide a history of each batch of product and of all circumstances pertinent to the quality of the final product.
Authorized Person
The person is recognized by the National Regulatory Authority as having the responsibility to ensure that each batch of finished products has been produced, tested and approved for release in accordance with the applicable laws and regulations in the country.
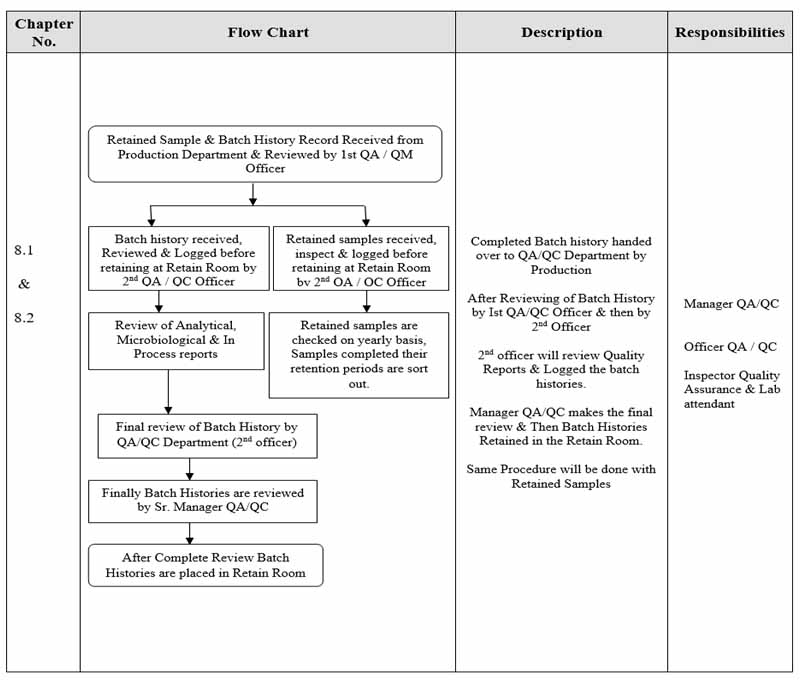
Flow Chart

Procedures
Sampling & Retention Of Reference sample
Surge & Customer Products:-
-
-
- Manager QA/QC designates the QA Officer/ QA inspector to draw the required quantity of sample of the finished product.
- The designated QA Officer takes the sample randomly during the packing of product from the packing line in the presence of Production officer and record the quantity sampled on batch document sheet.
- QA Officer Keep the samples in “Retained Sample & Batch History Room” in allocated racks as per master list and record it in “Batch History & Retained Sample Register”.
- The samples are stored below 30°C and at Humidity (NMT 65%).
- The retained samples are kept for 1 year after expiry date of the batch.
- Samples are physically examined after every 6 months for deterioration.
- Issue Retained Samples on “QA Records & Sample Issuance” Form
- Retention samples of materials and products should be of a size sufficient to permit at least two full re-examinations.
-
Raw Material Sample Retention:-
-
-
- Active Raw Material should be stored for Five (5) Years, starting from the GRN date.
- Material other than active should be stored for minimum of Two Years
- Raw Material should be stored in proper container with proper identification, including GRN number, Manufacturing Date, Expiry Date GRN Date & RM number (Quality Control number)
-
Regulatory Authority Sample:-
Whenever receive the sample from Regulatory Authority update the log and retained the sample in allocated area.
Receiving, Review, Retention & Issuance of Batch History Record
Receiving of Batch History Record
-
- Complete Batch History after the completion of batch and forward to QA/QC Department for Liquid Injectables, Dry Powder Injectables & Semi-Basic Manufacturing Area.
- Officer QA receives batch history & records it in “Batch History Record Receiving Register”
- Batch History Records are entered as sequential number & also note the Mfg Date, Exp Date & Receiving Date.
- Take the signature of that person who receives the batch history from QA Department.
- Officer QA ensures receiving of Batch history Records by signing the Production copy & “Batch History Record Receiving Register”
- Ensure the following before submission of Batch History Record:-
-
- Avoid over writing
- Avoid Cutting if required draw a line on already written text as it is visible & write NA with it as mentioned reason of change, then must be signed & dated as per Good Documentation Practices.
- Mention “Not Applicable” (NA) if space is not used at any step, signature & Date.
- Avoid any back dating on record
- Use of correction pen is not allowed
Review of Batch History Record
- Critically review batch historyIf any Non-Conformance observed, return batch history record to Production department to take corrective action. Production department takes corrective actions & returns the same.
- If batch history is complete then forward to Quality Control Department. Quality Control department add in process, final control analytical reports & also microbiological reports in batch history record, and return to QA Officer.
- After reviewing, Officer QA verifying by signing the completion of batch history and forwards to Sr. Manager QA/QMR.
- Manager QA/QMR ensures by sign all records filled, signed and filed properly in Batch History Record envelop.
- Production and quality control records should be reviewed as part of the approval process of batch release. Any difference or batch failure to meet the specifications must be thoroughly investigated. Investigation must, if necessary, extend to other batches of the same product and other products that might be related to failure or specific differences. The written record of the investigation must be carried out and must cover conclusions and follow-up actions.
- Records should be kept in such a way that activities concerning the production and quality control of active pharmaceutical ingredients are traceable.
- Batch Histories should be completed as the process going on and submitted to QA department as the batch completed. Batch should not be released until the batch history is found completed & satisfactory
- Handling of Retained Samples & Batch History Records is helpful in review of batch history records
Batch History Record of Ampoule & IV Infusion Comprises of the following
- Batch Manufacturing Order
- Material Dispensing Sheet
- Standard Manufacturing Procedure
- Ampoule / Vial (IV Infusion) Washing Record
- Line Clearance as per SOP of Line Clearance
- Sterilization Cycle Log
- Sterilization Load Verification Checklist
- In process Control Records
- Cleaning of Machine Equipment/Area
- Work Environment Control Record
- Product Volume Control Sheet
- Sampling Intimation Slip
- Product Optical Inspection Record
- Material Disposal note
- Quality Control Reports for Various Stages
- Batch Packaging Order
- Specimen of Label, Unit Carton and Leaflet signed by Production and QA
- Packaging Material Overprinting Record
- Digits (Stereos) of Coding
- Line Inspection Sheet
- Stock Transfer Ticket
- Return of Packaging Components
B) Batch History Record of Vial Comprises of the following
- Batch Manufacturing Order
- Material Dispensing Sheet
- Standard Manufacturing Procedure
- Vial Washing Record
- Line Clearance as per SOP of Line Clearance
- Sterilization Cycle Log
- Sterilization Load Verification Checklist
- In process Control Records
- Cleaning of Machine Equipment/Area
- Product Yield
- Work Environment Control Record
- Product Weight Control Sheet
- Sampling Intimation Slip
- Product Optical Inspection Record
- Material Disposal of retains sample.
- Quality Control Reports for Various Stages
- Batch Packaging Order
- Specimen of Label, Unit Carton and Leaflet signed by Production and QA
- Packaging Material Overprinting Record
- Digits (Stereos) of Coding
- Line Inspection Sheet
- Stock Transfer Ticket (STT)
- Return of Packaging Components
- Product Labeling Check List
C) Batch History Record of Semi-Basic Comprises of the following:-
- Batch Manufacturing Order
- Standard Manufacturing Procedure
- Fluid Bed Processor Parameters Monitoring Record
- Batch Packing Order
- Quality Control Release
Retention of Batch History Record
- Officer QA keeps the Batch History Records in “Retained Sample & Batch History Room” in allocated racks as per master list and record it in “Batch History & Retained Sample Register”
- Current Batch History Record must be located on top of the stack.
- The retained Batch History Records are kept for 1 year after expiry date of the Batch History Records.
- Retention period may be extended on the basis of technical decision and any critical complaint to particular batch.
- Dispose off Batch History Record after recommended period.
- Retentions Handling of Retained Samples & Batch History Records
Issuance of Batch History Record
- Issue Batch History Record for reference use after request on “QA Records & Sample Issuance”
Records
- QA Records & Sample Issuance
- Batch History Record Receiving Register
- Batch History & Retained Sample Register
- Master List of Handling of Retained Samples & Batch History Records
Reference
There are two reference of Handling of Retained Samples & Batch History Records as given below
Pharmaceutical Inspection Convention, Pharmaceutical Inspection Co-operation Scheme (PIC/S). In: Guide to good manufacturing practice for medicinal plants, Geneva, PIC/S Secretariat, 2000.
Quality Assurance of pharmaceuticals. A compendium of guidelines and related materials. Volume 2. Good manufacturing practices and inspection. Geneva, World Health Organization, 1999,
Distribution:-
Handling of Retained Samples & Batch History Records article are distributed in below mentioned Departments:-
1 Quality Assurance Department
2 Quality Management Department
3 Quality Control Department
Handling of Retained Samples & Batch History Records importance
Handling of Retained Samples & Batch History Records is import for pharmaceuticals because in future many time retained samples are used. Handling of Retained Samples & Batch History Records is workable when sample of any durgs are retested.


nothing special
how
please explain
very interesting, but nothing sensible
_________________
игровые автоматы играть на деньги скачать на телефон
Thanks, I’ve been looking for this for a long time
_________________
весь список казино онлайн
Thank you for your response to my blog https://www.pharmaegg.com
I look forward to continuing our relationship together.
Стремитесь улучшить свои английский? Погрузитесь в увлекательный мир английского языка через фильмы и сериалы на английском и британском языках. Насладитесь процессом обучения, погружаясь в увлекательные сюжеты и диалоги на языке оригинала. Фильмы и сериалы на английском не только помогут вам расширить словарный запас, но и улучшат понимание английской языка на слух. Используйте просмотры сериалов на английском в качестве занимательного метода изучения. Подробнее на сайте https://serialy-na-angliiskom.pp.ua.
Thank you for your response to my blog https://www.pharmaegg.com
I look forward to continuing our relationship together.
thanks, interesting read
_________________
играть в казино онлайн на деньги за бонус при регистрации