
Levosulpiride Raw Material Testing Procedure
Description
(Specification: White to off white powder)
Procedure
Spread approximately 2.0 gm of the sample over the cleaned and dried petri dish and examine visually against white background. Check the appearance of colour, nature and any visible foreign particles.
Solubility
(Specification: Freely soluble in dimethylformamide, sparingly soluble in methanol and slightly soluble in alcohol and in Methylene chloride).
Procedure:
Weigh the sample in specified quantity and transfer into a test tube. Add specified volume of the solvent shake for few minutes. Observe and record its solubility.
Identification
A) By Melting Range: Between 182°C to 186°C.
Procedure:
Crush the substance to very fine powder charge a capillary glass tube, one side sealed, with a sufficient amount of a powder to form a column in the bottom of the tube 25 to 35mm height packed down as closely as possible by moderate tapping in a solid surface.
Adjust the temperature of apparatus about 10°C below the melting point of the compound.
Insert the capillary at the sample point provided, when the temperature is about 5°C below the lower limit of the expected melting point.
Adjust the rate of heating 1 °C/min, and record the temperature at which the sample completely melts.
B) By IR
Take the background spectrum.
Place the reference or working standard substance to be examined in close contact with an Internal Reflection element (IRE) i.e. diamond. Ensure close and uniform contact between the substance and the whole crystal surface of the IRE by applying pressure. Examine the attenuated total
reflectance (ATR) spectrum in the region 400 cm-1 to 4000 cm-1•
Examine the ATR spectrum of test substance as in same manner of working standard.
C) ByTLC
The intensity of the spot obtained from test solution corresponding to standard should have the same intensity.
- Mobile Phase: Acetonitrile(50): Methanol (15): Formic acid (06).
- Test Solution: 100mg of test sample in 50ml methanol.
- Reference solution: Dissolve 100mg of reference standard in 50ml methanol.
Procedure
Apply separately 5 µl each of the test and the reference solution on the chromatographic plate. Run the plate in the mobile phase till the solvent front moves for about 8 ems from the point of application, remove the plate from the developing chamber and dry it in air. Observe the plate under UV light at 254nm.
D) By Fluorescence test
Chemicals Used: Sulphuric acid, Formaldehyde solution (37 % w/v)
Procedure
Place about 1.0 mg of sample in a porcelain dish.
Add 0.5 ml of sulphuric acid and 0.05 ml of formaldehyde solution.
Examined in ultraviolet light at 365 nm, the solution should show blue fluorescence.
Specific Optical Rotation
(Specification: Between (-66° to -69°)
Procedure
Dissolve 1.0 gm of the sample in 100 ml of dimethyl formamide.
Thermostatically adjust the temperature of the polarimeter tube and fill with dimethyl formamide. Insert in the polarimeter, wait until the temperature has equilibrated (5 min) and then determined the angle of rotation, and zero the value if necessary (blank).
Repeat the same procedure using the sample and calculate specific optical rotation at the wavelength of sodium light (D-line 589 nm) at 25°C ± 0.5 °C.
Angle of rotation x Capacity of volumetric flask in ml x 100
SOR ——————————-·————————————-·—
Sample weight in gm x Tube in length in decimeter x (100-¾LOD/¾water content)
Loss on Drying
(Specification: Not More Than 0.5% w/w)
Procedure
Dry the precleaned LOD bottle in oven for 30 minutes at 105°C ± 2°C and allow it to cool for 30 minutes in desiccator. Weigh the empty glass stoppered LOD bottle and record its weight as (A). Transfer 1.0 gm of sample in a LOD bottle; cover it with lid and accurately weigh the LOD bottle and record the weight as (B). Find out the sample weight (C) by (B-A). Close with lid and gently sidewise shake the bottle to distribute the sample uniformly, keep it in the drying oven, open the LOD bottle, and keep aside lid, dry the sample at 105°C ±2°C for 3 hrs. Take out the LOD bottle after closing the lid and keep it in desiccator. Allow it to cool for 30 minutes. Weigh the bottle and record the weight as (D). Find the loss in weight of Sample after drying as (E) by (B-D).
Calculate the loss on drying by given formula:
EX 100
Loss on drying = ————–
C
Sulphated Ash
(Specification: Not More Than 0.1 ¾w/w)
Procedure
Ignite a crucible (silica, platinum) at 600°C ± 50°C for 30 minutes, allow to cool in a desiccator over silica gel and record the weight as (A).
Place 1.0 g of the substance being examined in the crucible and record the weight as (B), found the sample weight as (C) by (B-A).
Moisten the substance to be examined with a small amount of sulfuric acid (usually 1 ml), heat gently at a low temperature as practicable until the sample is thoroughly charred.
Cool and then moisten the residue with a small amount of sulfuric acid heat gently until white fumes are no longer evolved and ignite at 600°C ± 50°C until the residue is completely incinerated Ensure that flames are not produced at any time during the procedure.
Allow crucible to cool in a desiccator over silica gel, record the weight as (D).
Found the residue weight as (E) by (D-A) and calculate the percentage of Sulphated ash.
Calculation
EXlO0
Sulphated ash (% w/w) = —————–
C
If the amount of residue so obtained exceeds the prescribed limit, repeat the moistening with sulfuric acid, heating and igniting as before using a 30 min ignition period until two consecutive weighing’s of the residue do not differ by more than 0.5 mg or until the percentage· of residue complies with the limit.
Chlorides
(Specification: Not more than 100 ppm)
Preparation of References Solution
Take 10 ml of 1000 ppm chloride standard solution into a 100 ml volumetric flask and dilute upto the mark with water (100 ppm Chloride standard solution).
Transfer 10 ml of above solution into a 100 ml ·volumetric flask and dilute upto the mark with water (10 ppm Chloride standard solution).
Preparation of Freshly prepared 5.0 w/v solution of silver nitrate.
Prepare a standard in the same manner using 10 ml of chloride standard solution and 5 ml of water.
Examine the tubes laterally against a black background
After standing for 5 min protected from light, any opalescence in the test solution is not more intense than that in the standard.
Procedure for Test
Shake 1.0 g with 20 ml of water. Filter through a sintered-glass filter (40). To 10 ml of the solution add 5 ml of water. Add 1 ml of dilute nitric acid (Dilute 20 g of nitric acid to 100 ml with water) Pour the mixture as a single addition into a test-tube containing 1 ml of silver nitrate solution.
Heavy Metals
(Specification: Not More Than 10 ppm)
Procedure
Lead Nitrate – Stock solution (100 ppm)
Accurately weigh 0.1598 gm of lead nitrate and transfer it into 1000 ml volumetric, add 100 ml of water and dissolve the contents. Add 1 ml of nitric acid and then make up the volume upto the mark with water. Prepare and store in glass containers free from soluble lead salts.
Standard Lead solution (10 ppm) to prepare just before use.
Take 10 ml of 100 ppm Lead standard solution into a 100 ml volumetric flask and dilute upto the mark with water (10 ppm Lead solution).
Preparation of Test Solution
Place 2 g of the substance to be examined in a silica crucible with 4 ml of a 250 g/1 solution of magnesium sulphate (MgSO4 H2O) in dilute sulphuric acid (IM). Mix using a fine glass rod.
Heat cautiously if the mixture is liquid; evaporate gently to dryness on a water bath.
Progressively heat to ignition and continue heat until an almost white or at most grayish residue is obtained.
Carry out the ignition at a temperature not exceeding 800°C. Allow to cool.
Moisten the residue with a few drops of dilute sulphuric acid (IM).
Evaporate, ignite again and allow to cool.
The total period of ignition must not exceed hydrochloric acid (2M).
Add 0.1 ml of phenolphthalein solution [0.1 % w/v in mixture of alcohol and water (80:20) then concentrated ammonia (13.5M) until a pink color is obtained Cool, add glacial acetic acid until the solution is decolorized and add 0.5 ml in excess.
Filter if necessary and wash the filter. Dilute to 20 ml with water.
Preparation of Standard Reference Solution.
Prepare as described for the test solution, using 2ml of lead standard solution (10 ppm Pb) instead of the substance to be examined.
To 10 ml of the solution obtained add 2 ml of the test solution.
Preparation of Monitor Solution
Prepare as· described for the test solution, adding to the substance to be examined the volume of lead standard solution (10 ppm Pb) prescribed for preparation of the reference solution To 10 ml of the solution obtained add 2 ml of the test solution.
Preparation of Blank Solution
A mixture of 10 ml of water and 2 ml of the test solution
To 12 ml of each solution, add 2 ml of buffer solution pH 3.5
Mix & add 1.2 ml thioacetamide reagent mix immediately. Examine the solutions after 2 min. The test is invalid if the reference solution does not show a slight brown colour compared to the blank solution or if the monitor solution is not comparable with the reference solution.
The substance to be examined complies with the test if any brown colour in the test solution is not more intense than that in the reference solution
If the result is difficult to judge, filter the solutions through a membrane filter (pore size 3 µm; without the pre-filter). Carry out the filtration slowly and uniformly applying moderate and constant pressure to the piston compare the spots on the filters obtained with the different solutions.
Related Substances (By HPLC)
Specification:
I-Octane Sul phonic Acid Sodium Salt Monohydrate (HPLC Grade)
Methanol (HPLC)
Acetonitrile (HPLC)
Orthophosphoric acid
Water (HPLC)
Buffer
Transfer 6.8 gm of potassium dihydrogen phosphate and lgm of sodium I-octane sulphonate in 1000 ml of water. Dissolve it and adjust the pH to 3.3 ± 0.1 with phosphoric acid. Filter and degas prior to use.
Mobile phase
Prepare mobile phase by mixing acetonitrile, methanol and buffer solution in the ratio of 100: 100:800 respectively.
Diluent: Mobile phase
Test solution
Transfer accurately 25 mg of sample in 25ml flask dissolve in and dilute it up to mark with mobile . phase.
Reference solution
Dilute 5.0 ml of the test solution to 100ml with mobile phase. Further dilutel.0 ml of the solution to 10 ml with the mobile phase.
Chromatographic system
- Column : Kromasil C18 column (250 mm x 4.0 mm x 5µm) or equivalent. ·
- Detector : UV
- Wavelength : 240 nm
- Flow rate : 1.5 ml/min
- Run time : 45 min.
- Injection Volume : 10 µI
Note: If required adjust the flow rate for getting the required retention time.
Note: Spike MSMB 10 ppm standard solution for RT conformation, if required.
Procedure
Set the chromatograph as described under chromatographic system. Inject 10µ1 of blank into the chromatograph until the baseline becomes stable. Inject reference solution into chromatograph & record the chromatogram then inject the test solution.
Examine the blank chromatogram for any extraneous peak & disregard any corresponding Peak observed in the chromatogram of sample solution.
The retention time of Levosulpiride is about 14.8 min.
The relative retention time of2-Methoxy-5-Sulfamoyl methyl Benzoate is about 0.5.
Calculate the percentage of each impurity using the formula
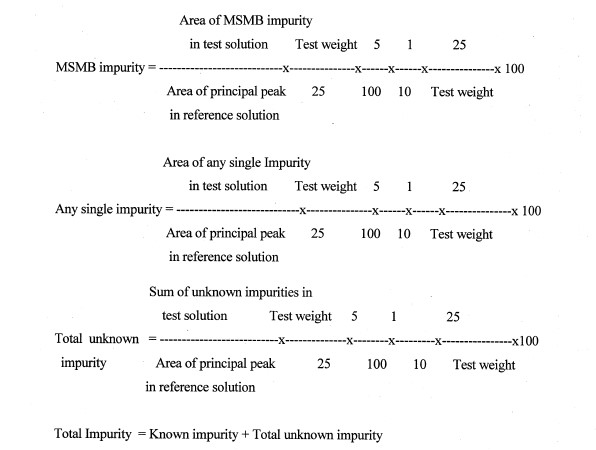
Assay (by Potentiometer)
(Specification: Between 98.5 % to 101.0%)
Procedure
Dissolve 0.250g in 50 ml of anhydrous acetic acid. Titrate with 0.1 M perchloric acid, determining the end-point.
Potentiometrically: Perform a blank titration and make necessary correction. 1 ml of 0.1 M perchloric acid is equivalent to 34.14 mg of C1sH23N3Q4S.
Residual Solvent (By GC)
Specification:
Ethylene Glycol: Not More Than 620 ppm
Methanol: Not More Than 500ppm
Apparatus
Analytical Balance
Gas Chromatography with Flame Ionization Detector
Reagents
Methanol – GC Grade
Ethylene Glycol – GC Grade
Chromatographic conditions for GC
i) Column: CP Sil 24 CB (30m x 0.53mm x 1.0 µm )or equivalent column
ii) Carrier gas: Nitrogen
iii) Flow rate: 4.2 ml/min
iv) Split Ratio: 5:1
v) Injection volume: 1 µl
vi) Inlet temperature: 225°C
vii) Run time: 24 min
viii) FID Temperature:300°C
ix) Hydrogen Flow: 40 ml/min
x) Air Flow: 400 ml/min
xi) Makeup Flow: 20 ml/min
xii) Equilibration time: 0.5 min
xiii) Oven program: Initial temperature 50°C hold for 0 min increased up to 240°C at the rate of 10°C and hold for 5 min.
xiv) Diluent: Acetonitrile
Test solution
Dissolved 0.500 gm of Levosulpiride in 5 ml acetonitrile.
Standard Solution
Weigh accurately 0.062 gm of Ethylene glycol and 0.05 gm of Methanol in 50 ml volumetric flask make up the volume with acetonitrile. Further dilute 1 ml of this solution to 20 ml with acetonitrile.
Procedure
Inject 1 µl of test and standards solution in duplicate in to the chromatograph and note down the area and calculate the content of each component in ppm using the following formula.
Set the chromatograph as described under chromatographic system. Inject blank (acetonitrile) followed blank record the chromatogram. Inject standard solution in duplicate. Then inject blank followed by sample solution in duplicate into the chromatograph and record the chromatograms. The retention time of Methanol is about 2.11 min.
The retention time of Ethylene Glycol is about 6.31 min.
Calculations
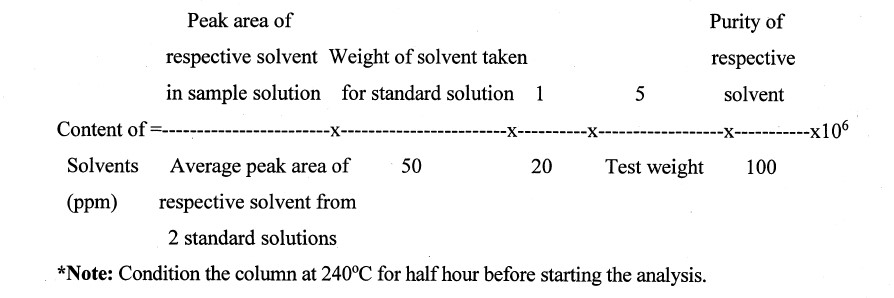
Particle Size by Malvern master size Specification:
d (90) : S25 µm
d (50) : S10 µm
Instrument parameters
- Range Length : 300RF mm.
- Beam Length : 2.40 mm.
- Presentation Mode : 3NJE
- Analysis : Polydisperse
- Sampler : MSI
- Dispersion Media : Paraffin liquid light.
- Stirrer Speed :2000RPM.
Procedure:
Wash the sampler with paraffin liquid light. The sampler with about 100 ml paraffin liquid light stirring and ensure that the obscuration is 0%.
Weigh about 30 mg of sample in a 25 ml beaker and add 10 ml of paraffin liquid light it.
Sonicate for 5 minutes with swirling.
Transfer the solution to sampler. So as to get obscuration of20-30%.
Stir the solution for six minutes and record the histogram.

