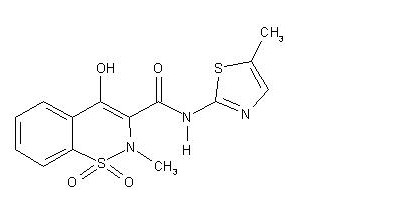
Meloxicam testing by UV method and In House method 2, meloxicam is used to relieve pain, tenderness, swelling, and stiffness caused by osteoarthritis (arthritis caused by the breakdown of the joint lining) and rheumatoid arthritis (arthritis caused by inflammation of the joint lining) Meloxicam 7.5mg Injection Testing by UV Method and In House Method is also used to relieve pain, tenderness, swelling, and stiffness caused by juvenile rheumatoid arthritis (a type of arthritis that affects children) in children 2 years of age and older. . Meloxicam is in a class of drugs called non-steroidal anti-inflammatory drugs (NSAIDs). It works by blocking the body’s production of substances that cause pain, fever, and inflammation.
PURPOSE
To describe the operational procedure Meloxicam 7.5mg Injection Testing by UV Method and In House Method to release the finished product for marketing.
SCOPE
This is applicable in all sections of the quality control department.
RESPONSIBILITY
- Lab Attendant is responsible for washing of the apparatus and cleaning the instruments.
- In–process Q.C pharmacist is responsible to do a sampling of the finished product.
- Q.C. Analyst is responsible to perform the test of the sample according to the procedures.
- Q.C. Incharge is responsible for an overview of the performance of the Q.C. Analyst.
MATERIAL & EQUIPMENT
Equipment:
- Analytical balance
- Sonicator
- pH Meter
- Spectrophotometer
- HPLC
Chemicals:
- Methanol
- 1M sodium hydroxide
- propan-2-ol
- Diammonium hydrogen orthophosphate
- Orthophosphoric acid
- 2-amino-5-methlthiazole
PROCEDURE:
TESTING ANALYSIS
- OPERATIONAL PROCEDURE
- Physical Analysis:
| PARAMETERS | SPECIFICATION |
| Physical Form | Injection |
| Color | Yellow color clear solution |
| pH | 10-12.5 |
| Wt/ml | 1.00-1.05 g/ml |
| Sealing | Should be properly sealed |
| Leaking | Should be leakage proof |
| Average Volume /Vial : | 50 ml |
CHEMICALS ANALYSIS:
( IN HOUSE Method )
Contents of Meloxicam:
Each ml contains 7.5 mg of Meloxicam
Assay:
Standard Preparation:
A. Weight accurately 7.5 mg of meloxicam reference standard and make the volume upto100 ml with methanol
B. Take 1ml from (step a) and make the volume upto 100ml with methanol
C. Measure the absorption of (step b) at 354 nm
Sample Preparation:
A. Take 1ml sample make upto 100ml with methanol
B. Take 1ml from (step a) and make the volume upto 100ml with methanol
C. Measure the absorption of (step b) at 354 nm
Calculation:
% age = Absorbance of Sample × 100
Absorbance of Standard
Reference:
(In House Method)
Limits : The contents of Meloxicam should be 90-110%
(Method NO 2)
Assay:
Carry out the method for liquid chromatography, using the following solutions. For solution (1) Take sample equivalent to 30 mg of meloxicam with 10 ml of 0.1 M sodium hydroxide, add 40 ml of methanol and mix with the aid of ultrasound for 5 minutes. Add a further 40 ml of methanol, mix for 3 hours using a magnetic stirrer and then with the aid of ultrasound for 5 minutes. Cool, and add sufficient methanol to produce 100 ml and filter. For solution (2) Dissolve 30 mg of meloxicam in 10 ml of 1 M sodium hydroxide and 40 ml of methanol, cool and add sufficient methanol to produce 100 ml. For solution (3) Dissolve 4.5 mg of 2-amino-5- methylthiazole in 20 ml of 1 M sodium hydroxide and 20 ml of methanol, cool and add sufficient methanol to produce 200 ml. Dilute 2 volumes to 100 volumes with methanol. For solution (4) mix equal volumes of solution (10 and solution (3).
The chromatographic procedure may be carried out using (a) a stainless steel column ( 10 cm * 4.0 mm ) packed with octadecylsilyl silica gel for chromatography ( 10 µm ) ( Kromasil 100 C 18 is suitable) maintained at 40°, (b) as the mobile phase with a flow rate of 0.8 ml per minute 370 volumes of a mixture of 650 volumes of methanol and 100 volumes of propan-2-ol and 630 volumes of a 0.20% w/v solution of diammonium hydrogen orthophosphate adjusted to pH 7.0 with dilute orthophosphoric acid and (c) a wavelength of 254 nm.
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution factor between the two peaks is at least 4.0.
Calculation:
%age = Area Covered by Sample
Area Covered by Std.
Reference:
British Pharmacopoeia
Limits : The contents of Meloxicam should be 95-105%

