Process change control is a crucial part of pharmaceutical manufacturing operations as it ensures that any modifications made to the manufacturing process are evaluated and documented to ensure product quality, safety, and efficacy. SOP for Procedure for Process Change Control is essential to follow a well-defined change control process in the pharmaceutical industry to ensure that any modifications made to the manufacturing process are thoroughly evaluated, documented, and controlled to ensure product quality, safety, and efficacy.
PURPOSE:
To control process variability
To operate the process smoothly
SCOPE:
This procedure is to be followed while controlling process change in the production department.
RESPONSIBILITY:
- Chief Executive Officer
- Quality Control Manager
- Quality Assurance Manager
- Inventory Control Manager
- Production Manager
- Maintenance Incharge
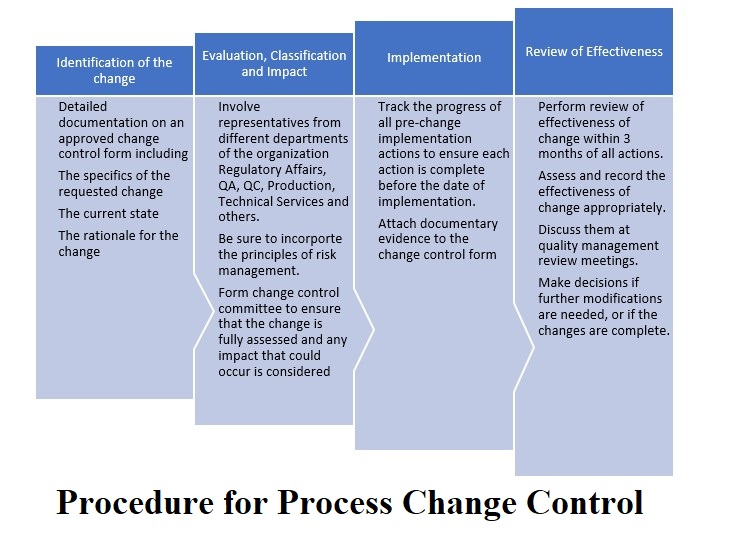
The following are the general steps for process change control in the pharmaceutical industry:
Initiation of Change: The first step is to identify the need for a process change. This could be due to various reasons such as process improvement, regulatory requirements, quality issues, equipment failure, etc.
Change Proposal: Once the need for a change is identified, a formal change proposal is initiated, which includes details of the proposed change, rationale, potential impact, and justification.
Evaluation of Change: A cross-functional team consisting of representatives from quality assurance, manufacturing, engineering, and regulatory affairs evaluates the proposed change. This team assesses the feasibility of the proposed change, potential risks, and impact on product quality.
Approval Process: Once the evaluation team completes the assessment of the proposed change, they submit the proposal to the change control board (CCB) for approval. The CCB is responsible for reviewing and approving all proposed changes to ensure that they comply with regulatory requirements and do not compromise product quality or safety.
Implementation of Change: Once the change is approved, it can be implemented. The implementation process should be carefully planned and executed to ensure that the change is properly documented and validated.
Post-Implementation Review: After the change is implemented, a post-implementation review is conducted to ensure that the change has been properly executed and that product quality, safety, and efficacy have not been compromised. Any deviations or issues that arise during this phase are documented and addressed.
Documentation and Record Keeping: All change control processes must be documented and recorded, including the change proposal, evaluation, approval, implementation, and post-implementation review. These records must be maintained for regulatory compliance and future reference.
Critical Process SOP for Procedure for Process Change Control :
Following are the critical processes in which change need control in order to control Process variability & for smooth operation:
- Change of source / supplier for starting & Packing Materials
- Change in standard Batch Size.
- Change in Product Master Formula.
- Abrupt change in running manufacturing / packing process.
- Batch break up during packing. / delay of packing of particular batch for More than 1 month.
- Any change in Engineering Process.
- Change in measuring & / or monitoring frequency.
- Any other change in Production process.
PROCEDURE:
CHANGE OF SOURCE / SUPPLIER OF STARTING & PACKING MATERIAL:
For manufacturing of injectables different type of starting martial actives like APIs & excipients etc, and different packaging components like Glass Vials, rubber stoppers, seals, labels, cartons etc are used and they are all purchased by different suppliers. The list of approved supplier is available at supply chain Department. If the supplier is to be changed following procedure will be followed.
Inventory Control Manager will intimate the Quality Control Manager and Quality Assurance Manager about supplier change on relevant Form. The approval will be taken about supplier change from Chief Executive on that form.
N.B. If the Product / process have been validated for the said supplier (Applied only in case of active pharmaceutical ingredient) the said product / process will be validated prior to commercial use of such material.
CHANGE IN STANDARD BATCH SIZE:
The standard batch size of each product are mentioned on product master
Formula document of each product. If a change is required in standard
Batch size the following procedure will be followed:
The competent authority (Chief Executive Officer) is only authorize to
Change the standard batch size. If the Batch size is changed to less than the standard batch size, the proposing person will intimate for said change to Quality Control Manager & Quality Assurance Manager for the said change on relevant form. The said change will be implemented after approval from Chief Executive.
If the change in batch size is higher than the standard batch size, the said change will be implemented after process validation of said product & approval from Chief Executive on relevant form.
CHANGE IN PRODUCT MASTER FORMULA:
If the product master formula of any product is to be changed due to any reason the change will be implemented after validation of that particular product / process & stability studies. The formal approval will be guaranteed from Chief Executive Officer.
ABRUPT CHANGE IN RUNNING MANUFACTURING / PACKING PROCESS:
If any abrupt change is required in manufacturing / packing process the proposed change will be intimated to Quality Control Manager & Quality Assurance manager on relevant form. The said change will be implemented after approval of Chief Executive on relevant form.
BATCH BREAK UP DURING PACKING / DELAY IN PACKING OF PRODUCT:
If a break up( mean partial packing) in particular batch is required the said change will be intimated to the Quality Control & Quality Assurance Manager by the person who has proposed the said change on relevant form.
The change is implemented after approval of chief Executive on relevant form.
ANY CHANGE IN ENGINEERING PROCESS:
If a necessary change in engineering process is required the maintenance Incharge will intimate the proposed change to production manager on relevant form . The production manager will discuss the said change in meeting of Validation committee. The said change will be implemented after approval of chairman of validation committee on relevant form.
CHANGE IN MONITORING & / OR MEASURING FREQUENCY:
If a change is proposed in measuring and / or monitoring frequency ( like calibration of gauges, environmental monitoring etc) the propose will intimate about the change on relevant form to the Quality Assurance & Quality Control Manager & the change will be implemented after approval of Chief Executive on relevant form.
ANY OTHER CHANGE IN PRODUCTION PROCESS:
If a change is required in manufacturing and / or packing process which is against the approved SMP, the incharge pharmacist will intimate the change to production manager. The said change will be communicated to Quality Assurance & Quality Control on relevant form by Production Manager. The change will be implemented after approval of Chief Executive on relevant form.
QUALITY FORMS AND RECORD
N/A
DISTRIBUTION
- Production Manager
- Assistant Manager Production
- Production Pharmacist
- Head of Operations
- QA Manager
- QC Manager

