
A Standard Operating Procedure SOP for Vendor Qualification in Pharmaceuticals Industry is a crucial document that outlines the steps and criteria for assessing and approving suppliers and vendors. This SOP ensures that the vendors selected meet the necessary quality and regulatory standards to maintain the integrity of pharmaceutical manufacturing processes. Below is a sample outline for a Vendor Qualification SOP:
PURPOSE:
The purpose of this SOP is to outline the procedure for screening of potential external providers / vendor’s and to assist in evaluation, pre-qualification, selection and reassessment of Raw, Packing and General Materials (Local & Import) supplies by developing enrich vendor database, uphold external provider/vendor information pool to develop partnering/long term Strategic Alliance.
SCOPE
- This SOP is applicable to procurement department to ensure the vendors pre-qualification/assessment process transparent, smooth and efficient.
- This document complies with WHO and PNAC (Pakistan National Accreditation Council) ISO/IEC 23025:2023(E) standard.
ROLE & RESPONSIBILITIES
| Roles | Responsibilities |
| Manager Supply Chain | |
| Quality Assurance Manager | |
| Assistant Manager Purchase | To issue the vendor registration form |
| Officer Procurement | |
| Officer Quality Assurance | |
| CEO/Director | Approval of Vendor Registration |
PROCEDURE
SOP for Vendor Qualification in Pharmaceuticals Industry
Pre-Requisites:
Following vendors/External Provider, categories shall be assessed and evaluated in the process of Pre-Qualification:
- Raw Material Manufacturers in Local Market
- Packing & Printing Material external provider/vendors
- International external provider/Vendors of Raw Material
- International external provider//Vendors of Packing Material
- General Items external provider/s/vendors
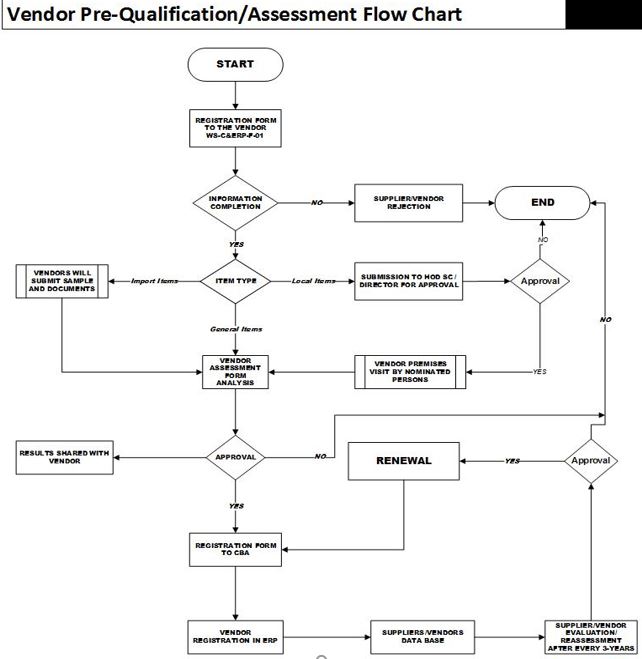
Assistant Manager Procurement / Procurement Officer shall issue the external provider/vendor Registration Form (QA-F-000) to the external provider/vendor and shall obtain information / clarification from vendor if needed.
If the information analysis given in the Registration Form (RF) results up to the mark, RF shall be forwarded to GM/Manager Supply Chain & Directors (if required) for approval.
International external provider/vendor’s will be requested to send relevant documents (as mentioned in the (Form# QA-F-000) along with pre-shipment “SAMPLE” of the materials. External provider/ supplier or Vendor shall be evaluated based on sample analysis.
- FOR API form API-S-01
- FOR Excipients From EXP-S-02
- FOR Packing Material form PM-S-03
Visits for International external provider/s/vendor site shall be made as per below:
- After analysis of provided documents, committee shall decide either to visit the external provider/vendor/ site or not.
- Committee shall be comprising on Head of Supply Chain & Manager Quality Assurance.
If the external provider/vendor/ is WHO, FDA, USDMF/CEP/API PQT/ API TDP Certified, or there are customer’s in Pakistan the visit is not mandatory.
After approval of external provider//vendor registration Form, # (QA-F-000) by CEO or Directors, nominated personnel from Supply Chain & Quality Assurance Departments shall visit vendor’s premises for assessment.
The nominated persons shall fill the external provider//vendor Pre-Qualification / Assessment Form # (QA-F-000) based on the observations during the visit to the vendor premises.
Filled Pre-Qualification Form # (QA-F-000) will be handed over to Manager Supply Chain after being signed by nominated persons from SCD and QA.
If circumstances are not appropriate i.e. strike, pandemic, emergency Desktop Audit would be performed.
In case of GENERAL item external provider/s/vendor premises shall be visited as per below:
- No visit shall be made at the external provider/vendor/s’ site who offer Shelf Items.
- Visit(s) shall be made only if external provider/vendor/ is the manufacturer of that particular item and the items has worth more than PKR, 50,000.
- The Registration Form # (WS-C&ERP-F-01) shall be signed by GM/Manager Supply Chain or authorized person nominated by GMSC (in case of General Items)
Evaluation / Re-Evaluation
- After approval of external provider/vendor based on Pre-qualification/Assessment (QA-F-000), Registration Form (QA-F-000) shall be handed over to Costing, Budgeting & Auditing (CBA) dept. for registration in SAP system.
- The external provider/vendors who are approved through Pre-Qualification/Assessment process shall be re-assessed after every three years.
- Assistant Manager Procurement shall be responsible to evaluate external provider/vendor triennially from July, 2023 on ERP base OOS Objection / Rejection / Complaint Report of vendor evaluation at the end of every third fiscal year (First evaluation will be done in July, 2026).
- Upon receipt of external provider//vendor’s evaluation report, Manager Supply Chain / QA Manager shall approve / disapprove the renewal by considering the results of evaluation report within 30 working days from date of inspection or audit.
- Assistant Manager Procurement shall be maintain detail of Vendor’s with date of approval/Evaluation & Re-evaluation.
- The evaluation outcome shall be shared with vendor as well by email or in person.
- External provider/Vendor’s re-evaluation/audit/Visit shall be made on risk analysis, Rejection and OOS Report, Change of premises or name, upgrade the machinery and equipment’s at plant.
Black Listing of Vendors:
- External Provider//Vendors may be blacklisted by Manager Supply Chain / QA Manager on the recommendation of procurement officer or above based on facts, which lead to financial loss / damage of the goodwill / reputation of the company.
- Below are three basic criteria for blacklisting of vendors:
- Three Consecutive rejection of item from egg QC department.
- Wastage quantity increases from agreed percentages during a reevaluation period.
- Increase in lead-time from the one agreed in the terms during an evaluation (except hold orders or agreed delivery date orders).
The information will be shared with all concerned stakeholders.
Switch to Alternate Source
- If Procurement / R&D need to switch to alternate source or add new source of Raw Material / packaging components, they are responsible to initiate the source approval request form based on the reasons given below in detail:
- Inability of a Source to consistently supply the material with agreed specifications.
- Failure to deliver the required ordered material in the required time frame (Delivery Problem).
- Discontinuation of manufacturing by existing Source due to any reason.
- Un-reasonably increase in material price (Cost issue).
- New Material for manufacturing of new product.
- Source does not update material specs as per current regulatory / Pharma copeial requirement.
- In case of new source / change source Procurement Representative following new vendor development procedure and complete the all pre-requisite
Vendor Approval Criteria
- Vendor acceptance criteria present in (QA-F-0000) for Vendor Approval.
- Acceptance Criteria is based on mutual scoring of QA, Procurement and R&D Department.
- An individual score of any vendor should not less than 70
| Acceptance Criteria | ||||||||||||||||||||||||||||||||||
| Any individual Score (i.e. Procurement, QA or R&D) Not Less Than 70.0 Final Rating Score = (Procurement + QA+R&D)/3 |
Final Rating Score | |||||||||||||||||||||||||||||||||
| 00.00 | ||||||||||||||||||||||||||||||||||
| Sr# | Final Rating Obtained Score | Description of Rating | REMARKS | |||||||||||||||||||||||||||||||
| I | 91-100 | Exceptional | ||||||||||||||||||||||||||||||||
| II | 81-90 | Good | ||||||||||||||||||||||||||||||||
| III | 71-80 | Satisfactory | ||||||||||||||||||||||||||||||||
| IV | 61-70 | Cautionary | ||||||||||||||||||||||||||||||||
| V | 51-60 | Unacceptable | ||||||||||||||||||||||||||||||||
FLOW CHART
GLOSSARY
SOP : Standard Operating Procedure
SCD : Supply Chain Department
PQ : Pre-qualification
QA : Quality Assurance
QC : Quality Control
PKR : Pakistani Rupees
R&D : Research and Development
CHANGE HISTORY
| DATE | Supersede SOP number | CLAUSE# | CHANGE MADE |
| — | This is the first version of this SOP |
RELATED DOCUMENTS
- External Provider/Vendor/ Registration Form (QA-F-000)
- Vendor Pre-Qualification/Assessment Form (QA-F-000)
REFERENCE
- PIQC
- ISO
- WHO Technical Report Series 961 , Point No. 8.6 Supplier/vendor management
DISTRIBUTION LIST
- Supply Chain
- Quality Control

