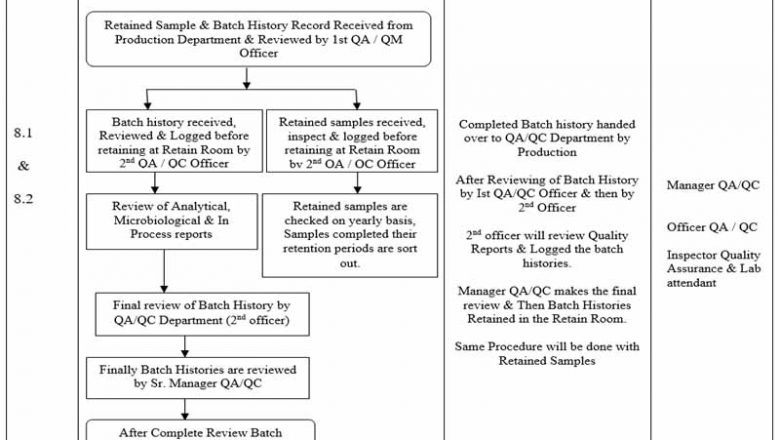
Inprocess Checking During Manufacturing SOPs
In post detail are given of Inprocess Checking During Manufacturing to maintained quality of all pharmaceuticals products.
Inprocess Checking During Manufacturing SOPs
To maintain and improve the quality of drug we need to Inprocess Checking During Manufacturing as per guidelines so that the potency of drugs increases.
Purpose:
This SOP "Inprocess Checking During Manufacturing" is designed to check the good manufacturing practices and Quality of all products during manufacturing (CGMP).
Scope:
This SOP "Inprocess Checking During Manufacturing" covers the all the Inprocess Checking During Manufacturing of solid Dosage forms that is Tablet and Capsule.
Responsibilities:
a. It is the responsibility of all Production Pharmacists, Production Staff and Q.A officers to follow this SOP dur...