
In this blog post, we are going to share the Vancomycin Hydrochloride Testing Procedure step by step. Vancomycin Hydrochloride is a very common drug, VANCOMYCIN HYDROCHLORIDE Intravenous use for Methycillin resistant staph aureus bloodstream infections.
Now we to our main topic Vancomycin Hydrochloride Testing Procedure Standard Analytical Procedure
APPROVAL BLOCK
| Title | Designation | Signature/Date |
| Written By: | Quality Control Analyst | |
| Reviewed By: | Quality Control Manager | |
| Verified By: | Quality Assurance In-charge | |
| Approved By: | Technical Operation Director |
Distribution List
| Sr. # | Department | New revision # | Retrieval Revision # | Signature & Date |
| 1 | ||||
| 2 |
DOCUMENT REVISION CONTROL
| Rev. # | Date | Initiated By | Page # | Nature of Amendment | Done By |
| 1 | |||||
| 2 |
1.0 OBJECTIVE:
To ensure chemical and microbiological analysis Raw Material VANCOMYCIN AS HCl dry powder for injection .
2.0 SCOPE
It is applicable in Quality Control Department chemical and microbiological analysis Raw Material VANCOMYCIN AS HCl dry powder for injection.
3.0 RESPOSIBILITY
- Q.C. Manager
- Microbiologist
- Quality Control Analyst
- Lab. Attendant
4.0 EQUIPMENT
- HPLC
- PH Meter
- Autoclave
- Petri dishes
- Incubator
- Hot plate
- Micro – pipette
- Sterilized borer
- Analytical balance
- Sterilized spatula/wire lope
- Conical flask 1000ml, 500ml, 250ml
- Graduated cylinder 100 ml (2)
- Beakers 100 ml,50ml,250ml, and 500ml
- Volumetric flask 100ml,500ml,100ml,50ml,250ml
- Graduate pipette 1 ml, 10ml, 2ml, 5ml (pair of each)
5.0 PROCEDURE FOR MICROBIOLOGICAL ASSAY :
5.1 Lab ware used for the storage and transfer of test dilutions and microorganisms must be sterile and free from interfering residues. Sterilize the glass apparatus in hot air oven for 01 hour at 180 °C.
5.2 Liquefy a medium suitable for the conditions of the assay and inoculate it at a suitable temperature, for example 48 °C to 50 °C for vegetative forms, with a known quantity of a suspension of micro-organisms sensitive to the antibiotic to be examined, such that clearly defined zones of inhibition of suitable diameter are produced with the concentrations of the antibiotic used for the assay. Immediately pour into Petri dishes or large rectangular dishes a quantity of the inoculated medium to form a uniform layer 2 mm to 5 mm thick.
5.3 Store the dishes so that no appreciable growth or death of the micro-organisms occurs before the dishes are used and so that the surface of the medium is dry at the time of use. Using the solvent and the buffer solution indicated in Table I , prepare solutions of the reference substance and of the antibiotic to be examined having known concentrations and presumed to be of equal activity.
5.4 Apply the solutions to the surface of the medium, for example, in cavities prepared in the agar. The same volume of solution must be added to each cavity. Same volume of reference solution must be added to each cavity. In order to assess the validity of the assay, use not fewer than 3 doses of the reference substance and 3 doses of the antibiotic to be examined having the same presumed activity as the doses of the reference substance.
5.5 It is preferable to use a series of doses in geometric progression.
Incubate at a suitable temperature for diffusion prior to incubation, usually 1 h to 4 h, at room temperature or at about 4 °C, as appropriate, may be used to minimize the effects of the variation in time between the application of the solutions.
TABLE I
| Antibiotic | Type of Assay |
| Vancomycin | Cylinder- Plate |
6.0 Preparation of Inocula
6.1 Spore suspensions of the organisms (Bacillus subtilis ) to be used as inocula are prepared as follows.
6.2 Grow the organism at 35-37 °C for 7 days on the surface of a suitable medium to which has been added 500MG /l of manganese sulphate R. Using sterile water R, wash off the growth, which consists mainly of spores.
6.3 Heat the suspension at 70 °C for 30 min and dilute to give an appropriate concentration of spores, usually 10 × 106 to 100 × 106 per milliliter. The spore suspensions may be stored for long periods at a temperature not exceeding 4 °C. Alternatively, spore suspensions may be prepared by cultivating the organisms in medium C at 26 °C for 4-6 days, then adding, aseptically, sufficient manganese sulphate R to give a concentration of 0.00 500MG /l and incubating for a further 48 h. Examine the suspension microscopically to ensure that adequate spore formation has taken place (about 80 per cent) and centrifuge. Re-suspend the sediment in sterile water R to give a concentration of 10 × 106 to 100 × 106 spores per milliliter, and then heat to 70 °C for 30 min. Store the suspension at a temperature not exceeding 4 °C.
7.0 Preparation of Sample:
Constitute a container of Vancomycin Hydrochloride with 10ml water for Injection.
Take 2 ml from the diluted vial and dilute it up to 100 ml with distill water to yield 1mg/mL of Vancomycin. This will be higher concentration of Test dilution. For making low dilution, take 25 ml from the first dilution and make the volume up to 50 ml with distill water.
8.0 Preparation of Standard:
Take standard equivalent to 500 mg of Vancomycin and dilute it with distill water to yield 1mg/mL of Vancomycin. This will be higher concentration of standard. For making low dilution, take 25 ml from the first dilution and make the volume up to 50 ml with distill water.
Medium A
- Peptone 6 g
- Pancreatic digest of casein 4 g
- Beef extract 1.5 g
- Yeast extract 3 g
- Glucose monohydrate 500MG
- Agar 15 g
- Water to produce 1000 ml
8.1 Well-Digging(cylinder) and Pouring:
Dig the 4 wells into solidified media in plate under sterile condition (under laminar flow) aseptically with sterilized borer (Cork Borer # 5). Pick and remove agar plugs from the digged wells with the help of sterile spatula or a sterile wire loop.
Pour reference sample dilutions (STDH and STDL) and Test Sample dilutions (TSH and TSL) in their respective holes, 50 µ liters each with micropipette.
Where:-
STDH = High Concentration of Standard Sample
STDL = Low Concentration of Standard Sample
TSH = High Concentration of Test Sample
TSL = Low Concentration of Test Sample
Calculations:
Calculate the contents as follows:
a (TSH +TSL) – (STDH + STDL) / (TSH +TSL) + (STDH – STDL) % potency = Antilog (2.0 + log I)
I = Ratio between dilution (2: 1)
Where 2.0 is a constant value.
Potency Limits: NLT 90 %.
IDENTIFICATIONS BY FTIR SPECTRUM:
Take the IR spectrum of the raw material sample, and it should be concordant with the spectrum of Working standard of Vancomycin Hydrochloride.
Sterility TESTS :
It meets the requirements when tested as directed for Test for Sterility of the Material To Be Examined, Membrane Filtration, except to dissolve the specimen in water, instead of in Fluid A.
BACTERIAL ENDOTOXINS TEST :
it contains NMT 0.33 USP Endotoxin Unit/mg of vancomycin.
• pH : 2.5-4.5, 50 mg/mL in water
• WATER DETERMINATION : NMT 5.0%
COMPOSITION OF VANCOMYCIN:
Solution A: Triethylamine and water (1 :500). Adjust with phosphoric acid to a pH of 3.2.
Solution B: Acetonitrile, tetrahydrofuran, and Solution A ( 7:1:92 )
Solution C: Acetonitrile, tetrahydrofuran, and Solution A (29:1:70 )
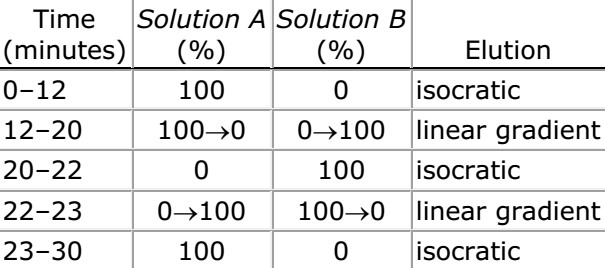
Mobile phase: See the gradient table below. [NOTE Make adjustments, if necessary, changing the Acetonitrile proportion in Solution B to obtain a retention time of 7.5- 10.5 min for the main Vancomycin peak.]

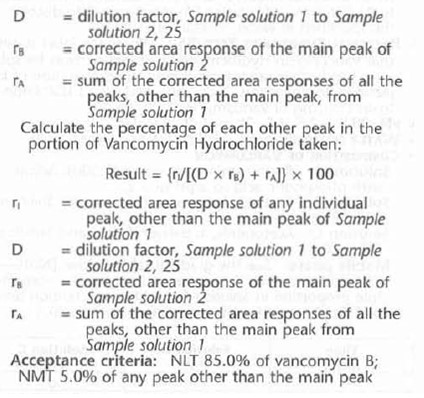
Description :
White, almost white or tan to brown powder
Visible Particulate Matter:
Reconstitute 2.5g in 50ml water for injection and perform optical checking. There will be no
Visible particle in any vial.
Sub Visible Particulate Matter:
Perform test of particle count of reconstituted vials with particle counter.
Particles should be in the following maximum range. If particles will out of this maximum
Range then Material will be rejected on the bases of this result.
> 10µm NMT 6000 per Vial , > 25µm NMT 600 per Vial
9.0 RISK ANALYSES:
EVIDENCE OF RECORDS & REFERENCES
USP44, NF39 Certificate of Analysis Raw Material
FORMAL KPIs (Key Performance Indicators)
Storage Condition Contamination of sampling tools
Disclaimer: We only share these posts for knowledge purposes and you can use it for your knowledge and pharma purposes but you’re prohibited to copy data for your website. If you want to use it then contact us or credit us in the post by giving our link. Thank you

