
A Standard Operating Procedure SOP for Change Control in Pharmaceuticals industry is a critical document that outlines the systematic approach for managing and documenting changes to processes, systems, equipment, facilities, or any other aspect that may impact product quality, safety, or efficacy. The following is a general outline for creating an SOP for Change Control in Pharmaceuticals:
Purpose
Scope SOP for Change Control in Pharmaceuticals
Responsibilities
- It is the responsibility of QA to prepare, manage and properly implement this SOP.
- It is the responsibility of all the employees, planning to introduce change(s) in already approved system follow this SOP as it is written.
- QA and initiator of change are responsible to conduct impact assessment of proposed change(s).
- QA is responsible to issue change control number, assign responsibilities, assure implementation of change(s) and do follow up as necessary.
- QA ensures that this SOP is followed in its entirety.
- It is the responsibility of Assistant QA Manager or Designee to review this SOP.
- It is the responsibility of QA Head or Designee to approve this SOP.
- It is the responsibility of Director Technical to authorize this SOP.
Definitions & Abbreviations
- Change: Any addition, deletion, or modification to approved documents, products, process, computerized system, utilities, facilities, equipment, instruments and vendors
- Change Control: It is a quality management tool to handle, maintain and keep tracking of all major / minor changes. It may be planned activity or forced changes that can be for shorter period of time or it can be permanent
- Major change: A change that may have an direct impact upon the product quality, process, safety / procedure
- Minor change: A change that may have no direct impact upon the product quality, process, safety / procedure
- Document Changes: Any changes on already approved GMP documents
- SOP: Standard Operating Procedure
- QM: Quality Management
- QSM: Quality System Manual
- SMF: Site Master File
- VMP: Validation Master Plan
- CCF: Change Control Form
- CC: Change Control
- cGMP: Current Good Manufacturing Practices
- APIs: Active Pharmaceutical Ingredients
- BMR: Batch Manufacturing Record
- BPR: Batch Packaging Record
- HOD(s): Head of Department(s)
- CCC: Change Control Committee
Departmental Codes
- QA: Quality Assurance
- QC: Quality Control
- PR: Production
- EG: Engineering
- AD: Administration
- PU: Procurement
- AC: Accounts
- IT: Information Technology
- WH: Warehouse
- RG: Regulatory Affairs
- RD: Research & Development
- EX: Export
Procedures SOP for Change Control in Pharmaceuticals
Types of Changes
A. Major Changes
- Change of manufacturer of APIs and Excipients.
- Shifting of processes to another site / place / section.
- Shifting of equipment(s) to another site / place / section.
- Major change in the product composition or master formula.
- Remarkable change in the process parameters.
- Changes to critical utilities.
- Changes in the manufacturing equipment.
- Changes of laundry for work cloth (Sterile).
- SOP for Change Control in Pharmaceuticals
B. Minor Changes
- Change of cleaning agent for floor
- Documents changes
Domains for Change Control
A. Manufacturing
- Shifting of whole manufacturing site / section to another site, place or section
- Major change in equipment
- Up gradation of equipment(s)
- Change in critical process control parameters
- Change in batch size
- Change in cleaning procedure of equipment
- Change in cleaning agent
- Change in environmental conditions
B. Engineering
- Major change in any equipment
- Modification in any equipment
- Change in any critical part of any equipment
C. Facility Design
- Up gradation of facility design
- Modification in Air handling unit system
D. Material Management
- Change in source of any Raw material or Primary packaging material
- Change in artwork of packaging material
E. Research Development
- Change in Shelf Life
- Change in Specifications
- Change in Composition
- Change in Storage Conditions
F. Quality Control
G. Quality Assurance
- Change in SMF
- Change in QSM
- Change in VMP
H. Documentation SOP for Change Control in Pharmaceuticals
- Change in SOPs, Forms, Templates
- Change in BMR and BPR
- Change in Validation / Qualification / Stability Protocols
- Any change in already approved documents
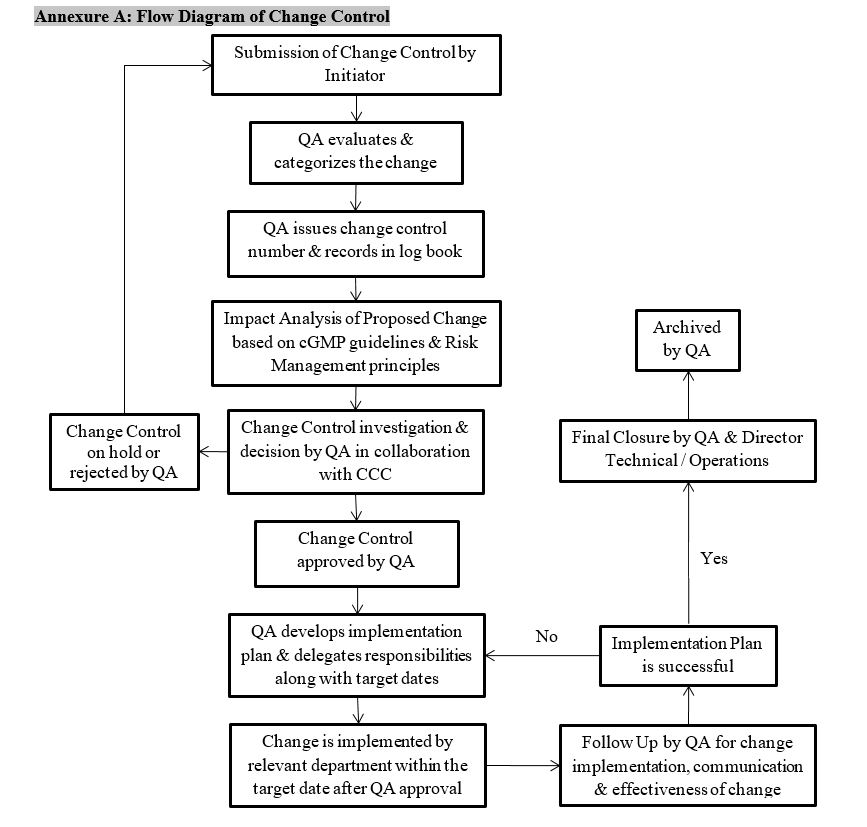
5.3 Initiation of Change Control (See Annexure A)
- Initiator can be user / controlling department / auditor / regulator or any relevant person
- HOD after consultation / recommendation and consensus with initiator, generates Change Control
- The HOD / initiator based on justification must submit the Change Control Form (QA/FRM/0000/00) to QA
- Initiator fills the form and makes available all documents related to proposed changes and all attachments forwards to HOD
- HOD forwards Change Control form to QA for its impact assessment and approval
- SOP for Change Control in Pharmaceuticals
| Raw material change | Any changes to raw material supplier, manufacturer processes, or storage of products |
| Packaging components | Any change to packaging components. This includes but not limited to: Bottles, caps, tubes, foils (thickness, materials dimensions), artwork |
| Manufacturing process change | Any changes to processing procedures. This includes but not limited to: 1. Manufacturing process (stirring time, blending time), order of addition, drying time, or temperature 2. Packaging process (cap torque, filling temperature, fill volume, blister seal temperature) 3. Cleaning process (time, temperature, method, cleaning agent) 4. Introduction of new product into manufacturing facility (if any). |
| Equipment /computer changes | Any modification or alteration to equipment, or the relocation of equipment from one place to another or the introduction of new equipment. This includes but not limited to: 1. Manufacturing equipment (manufacturing and storage tanks, blender, stirrers, compression machines, drying oven, granulation, FBD) 2. Purified water system (pump, valve, dosing machine 3. Packaging equipment (labeller, shrink wrapper,) 4. Testing equipment, scale, HPLC, balance,) pH, viscometer, spectrophotometer) 5. Environmental (air conditioning, temperature, humidity, pest control, |
Change Control Numbering and Recording System
- QA assigns Change Control No. and records in Change Control Log Book (Annexure B)
- The change control numbering system is based on the following format:
CC / DD / YY / 0000
where;
CC: Change Control
DD: Departmental Code
YY: Year
0000: Sequential Number of Change Controls per annum
Example 1: CC / PR / 23 / 0001: The 1st Change Control initiated by Production Department in the year 2023
Example 2: CC / QC / 23 / 0005: The 2nd Change Control initiated by Quality Control Department in the year 2023
Approval and Review of Change Control
- QA head reviews the form, reviews all attached documents and planned programs related to changes
- QA along with CCC analyze the impact factor and evaluates either changes to be done or not
- After decision, QA categorizes the changes and approves as per category
- After a successful review, QA approves the Change Control and issues the change control number
- After approval, duties are assigned to responsible persons and change is implemented by the relevant department within the target date
- In case change control is related to regulatory, prior approval from Director Technical / Operations and QA Head is compulsory
Note 1: No Major change is acceptable unless approved by Director Technical / Operations
Note 2: Mention No. in the form, if change control is on the basis of Complaint No. / CAPA No. / Deviation No. / OOS No.
Evaluation and Implementation SOP for Change Control in Pharmaceuticals
- QA Head assigns the effective date of implementation of the changes and the originating department plans for any necessary training and update the relevant documents with corrective actions jointly with QA
- The status of change and completion of action is mentioned in the change control form by the originating department along with QA
- After evaluation, the documents undergoing for changes / revisions are revised as per the requirement
- Upon completion of implemented change, QA follows up on implemented change to determine whether or not actions taken were effective and that the resulting change was communicated to all concerned parties
Note: All changes which can influence on the attributes of a GMP-relevant system, facility, equipment, material / product, and procedure / process should formally be requested by employees. The Change Control Form must be signed by QA Manager
Change Control Committee
- On the basis of impact on product quality, regulatory requirements and safety of personnel assessment, the acceptance or rejection of proposed Change is decided by the QA along with CCC
- CCC itself can initiate Change Control, in this case it can be directly proceeded by the nominated member, selected by the CCC
- Composition of CCC
• Director Technical / Operations
• QA Head
• HOD
• Initiator of that department
• HOD of any other relevant department (if required) - Change must be communicated to all the relevant persons and departments through E-mail
- CCC organizes meeting either in the presence of Directors or intimates Directors regarding change prior to final decision and implementation
Follow Up and Final Closure
- Change control must be close within the target date, if exceeding a proper justification to be presented
- Measurement of Change Effectiveness: Assess the measure of effectiveness by performing the followings checks:
• Monitor periodically to determine the implemented changes were effective
• Monitor periodically to confirm the problem is resolved - QA assesses the effectiveness of implemented changes periodically to confirm that the problem is resolved and change is productive
- Final Closure of Change Control is done by GM QA or Designee And Director Technical / Operations
- Change Control documents are archived by QA
Revalidation
- Revalidation should be carried out following a change that could have an effect on process, procedures, and quality of products and/or of the product characteristics
- Revalidation should be considered as part of change control
- Changes that are likely to require revalidation includes following but not limited to:
• Changes in manufacturing process (e.g. mixing time, drying temperature)
• Changes in equipment (e.g. addition of automation detection system)
• Production area and support system changes (e.g. rearrangement of area or a new water treatment methods)
Quarterly Change Control Review
- The effectiveness of the change control system is to be determined on quarterly basis in Quality Council Meeting
• Total number of Change Control till date
• Total number of closed Change Control till date
• Change type / department involved
• Time duration of procedure from initiation to closing
• Total number of internal or external complaints leading to change control
Attached Documents (Forms / Annexure)
- Flow Diagram of Change Control (Annexure A)
- Format of Change Control Log Book (Annexure B)
- Change Control Form (QA/FRM/0000/00)
Reference & Linked Documents
- ICH, Q10 Pharmaceutical Quality System (ICH, June 2023)
- European Commission, EudraLex Volume 4, Part I, Chapter 4 Documentation
- SOP for Management of Documents
Revision History
| Revision No. | Date | Responsible Person /Designation | Description of Change |
Distribution List
| Recipients | Copy Number | Issued on (Signature / Date) | Withdrawn on (Signature / Date) |
| Quality Assurance | Master | ||
| Quality Assurance | 01 | ||
| Quality Control | 02 | ||
| Production | 03 | ||
| Engineering | 04 | ||
| Administration | 05 | ||
| Procurement | 06 | ||
| WHD Raw Material | 07 | ||
| WHD FG Store | 08 | ||
| IT | 09 | ||
| Accounts | 10 | ||
| Export | 11 | ||
| Regulatory Affairs | 12 | ||
| Research & Development | 13 |
Annexure A: Flow Diagram of Change Control
Annexure B: Format of Change Control Log Book
| Section A To be completed by Change Initiator Date: ______________________________ Name of Change Initiator: _________________ Designation / Department of Change Initiator: ________ Nature of Change: Document System Facility VendorEquipment / InstrumentOther(s) __________________________ Description of change:____________________Justification for change:_____________________Change Priority: High Medium Low Proposed Implementation Date: ______________________________ Head of Department (Sign. / Date): _____________________________ |
||||||||||||||||||||||||
|
Section B To be completed by QA Change Control Number: _____________________ Category of Change: Major Minor If change control is on the basis of Complaint No. / CAPA No. / Deviation No. / OOS No. mention No.:________________ Impact Assessment: _______________________ Requested change is: Approved Hold Rejected Change Control Committee: |
||||||||||||||||||||||||
|
Section C To be completed by QA List of Areas Impacted by the Change 1) ___________________________ 2) _________________________ 3) ___________________________ 4) _________________________ List of Documents Impacted by the Change 1) ___________________________ 2) _________________________ 3) ___________________________ 4) _________________________
QA Manager or Designee: ______________________ Change Implemented Successfully Yes No |
||||||||||||||||||||||||
| Section D Follow Up & Final Closure 1. Implemented change was effective/ problem resolved Yes No2. Implemented change was productive Yes No3. Implemented change resulted in improvement Yes No Provide reasoning if action taken not effective / did not resolve the problem:____________ List of Concerned Departments 1) ___________________________ 2) _________________________ 3) ___________________________ 4) _________________________ 5) ___________________________ 6) _________________________ Conclusion: ________________ Change Control Closed On: ______________ GM QA or Designee: ____________________ Director Technical / Operations: ______________ |

