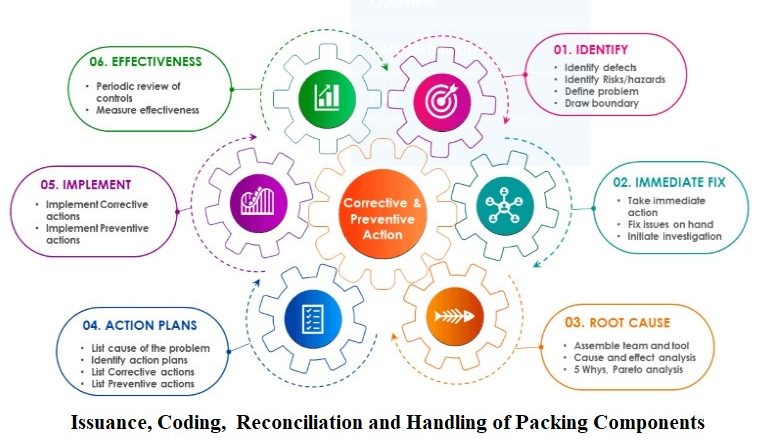
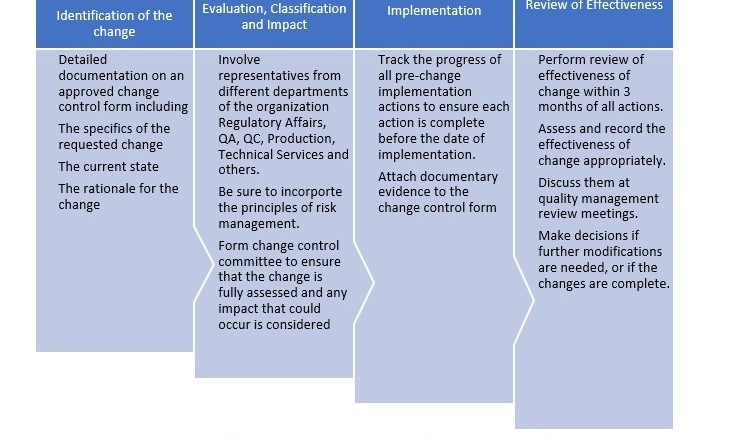
Trimetazidine Hydrochloride MR 35mg Tablet Testing Procedure SOP
Trimetazidine Hydrochloride MR 35 mg Tablet assay testing is a process to determine the potency of the active ingredient in the medication. This Trimetazidine Hydrochloride MR 35mg Tablet Testing Procedure SOP ensures that the medication contains the correct amount of the active ingredient, trimetazidine hydrochloride, which is necessary for it to be effective.
PURPOSE:
To describe the procedure for analysis at in-process and finished stage of Trimetazidine Hydrochloride MR 35 mg Tablet.
SCOPE:
This SAP gives a detailed outline for the finished product analysis of Trimetazidine Hydrochloride MR 35 mg Tablet and will cover In process testing activities on a physical, chemical & instrumental basis.
RESPONSIBILITY:
QC Analyst is responsible for physical / chemical testing and prepar...