How to Test Xanthan Gum Raw Material
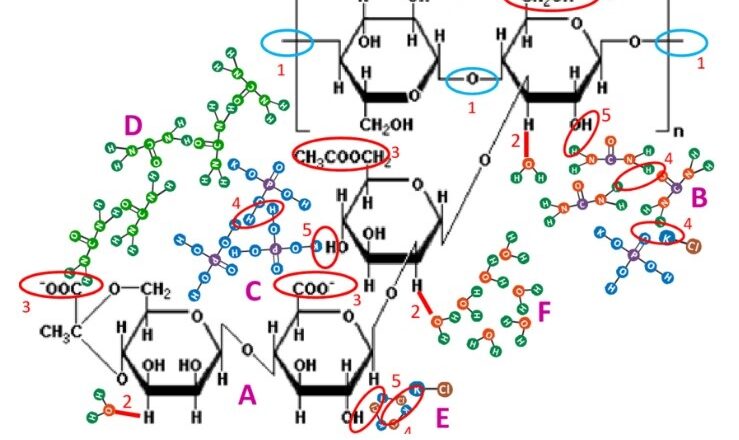
It is important to How to Test Xanthan Gum Raw Materials to follow industry standards, such as those set by the American Oil Chemists' Society (AOCS), when testing xanthan gum. Xanthan Gum is a high molecular weight polysaccharide gum produced by a pure-culture fermentation of a carbohydrate with Xanthomonas campestris, then purified by recovery with Isopropyl Alcohol, dried, and milled. How to Test Xanthan Gum Raw Material contains D-glucose and D-mannose as the dominant hexose units, along with D-glucuronic acid, and is prepared as sodium, potassium, or calcium salt. It yields NLT 4.2% and NMT 5.0% of carbon dioxide, calculated on the dried basis, corresponding to NLT 91.0% and NMT 108.0% of Xanthan Gum.
Purpose
To ensure the quality of incoming raw material of Xanthan Gum.
Scope
It ...