The testing of Molnupiravir raw material can be done through various methods, How to Test Molnupiravir (Method of Analysis) including High-Performance Liquid Chromatography (HPLC): This method is used to determine the purity and potency of the raw material, Nuclear Magnetic Resonance (NMR) Spectroscopy: This method is used to identify the structure of the raw material, Infrared Spectroscopy (IR): This method is used to identify functional groups in the raw material, Mass Spectrometry (MS): This method is used to determine the molecular weight of the raw material, X-Ray Crystallography: This method is used to determine the crystal structure of the raw material.
It is important to follow good manufacturing practices (GMP) and standard operating procedures (SOPs) during the testing process to ensure accurate results.
Abstract
An accurate, sensitive, and selective RP-HPLC-UV method for estimating Molnupiravir (MOL) in pure bulk powder and pharmaceutical formulation has been developed. Separation was accomplished on an Inertsil C18 column (150.0 mm 4.6 mm, 5.0 m) in isocratic mode with a mobile phase of 20 mM phosphate buffer pH 2.5[thin space (1/6-em)]:[thin space (1/6-em)]acetonitrile (80[thin space (1/6-em)]:[thin space (1/6-em)]20, v/v%). MOL prepared in the chosen diluent (ethanol[thin space (1/6-em)]:[thin space (1/6-em)]water in equal proportions) had a max of 230.0 nm. The calibration curve that was created was found to be linear in the concentration range of 0.2-80.0 g mL1. The proposed method resulted in a MOL recovery rate of 100.29%. The detection limit (LOD) and the limit of quantification (LOQ) values were 0.04 and 0.12 g mL1, respectively. In the presence of common pharmaceutical formulation excipients, no significant interference was detected. The method was validated in accordance with the ICH recommendations. All of the obtained results were statistically compared to those obtained using the reported methods, and no significant differences were found. The method developed in this study was successfully used to evaluate MOL in bulk powder and pharmaceutical formulations.
Analytical Procedures
Characteristics
Appearance
Visual inspection, this product is white to off-white powder.
Identification:
HPLC method
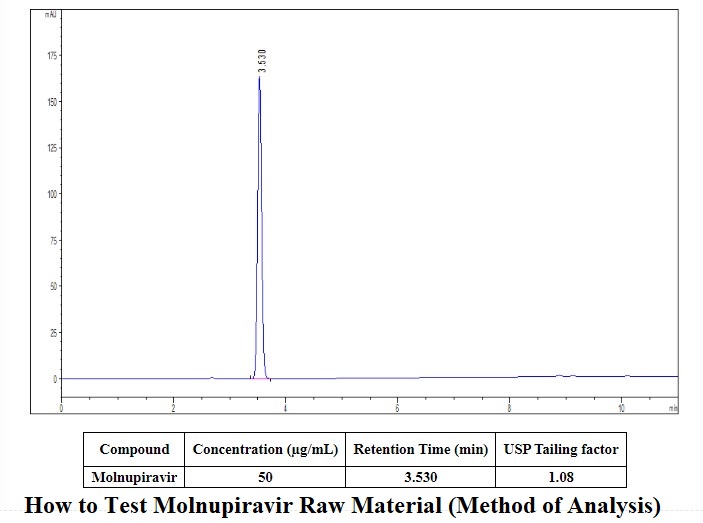
How to Test Molnupiravir (Method of Analysis) in the chromatogram recorded under the assay item, the retention time of the main peak of the test solution should be consistent with the retention time of the main peak of the reference substance.
Infrared spectroscopy
The infrared absorption spectrum of this product should be consistent with that of the control (Chinese Pharmacopoeia 2020 Edition General Principle 0402).
Water
Take this product and check it according to law (Chinese Pharmacopoeia 2020 Edition General Rules 0832), the moisture content should not exceed 1.0%.
Sulphate
Standard: 100μg/ml SO42-
Take this product 1.0g and check it according to law (Chinese Pharmacopoeia 2020 Edition General Rules 08012), which shows no more sulfate than corresponds to 1.0 mL standard solution (0.02%).
Chloride
Standard solution: 10μg/ml C1-
Take this product 0.5g and check it according to law (Chinese Pharmacopoeia 2020 Edition General Rules 0801), which shows no more chloride than corresponds to 4.0 mL standard solution (NMT 0.02%).
Residue on ignition
Take 1.0g of this product and check it according to law (Chinese Pharmacopoeia 2020 Edition General Rules 0841), and the remaining residue should not exceed 0.1%.
Heavy metals
Take the residues left under the ignition residues and inspect them according to law (Chinese Pharmacopoeia 2020 General Regulations 0821). The content of heavy metals must not exceed 20parts per million.
Related substance
Take about 10mg of this product, weighed accurately, put into a 10ml measuring bottle, add an appropriate amount of solvent ( 10% acetonitrile ) to dissolve and dilute to the mark, shake well, as the test solution; Precisely take 1ml of the test solution, dilute with solvent ( 10% acetonitrile ) to make a solution containing about 1μg per 1ml, as a control solution. Chromatographic conditions and system suitability tests used octadecylsilane-bonded silica gel as a filler; 0.1% phosphoric acid aqueous solution ( 5mol/L sodium hydroxide solution adjust the pH value to 3.0 ) was used as the mobile phase A, and acetonitrile was used as the mobile phase B; Linear elution was performed according to the gradient elution procedure. The flow rate is 1.0ml per minute; the detection wavelength is 230nm; the column temperature is 30 ℃; accurately measure 10 µl of the test solution and the control solution respectively, and inject them into the liquid chromatograph respectively, record the chromatogram. If there is an impurity peak or a single impurity peak in the chromatogram of the test solution. The single impurity area should not be greater than the main peak area of the control solution ( 0.1% ) , and the sum of the areas of the impurity peaks should not be greater than 10 times of the main peak area of the control solution ( 1.0% ).
| Time(min) | Mobile phase A(%) | Mobile phase B(%) |
| 0 | 90 | 10 |
| 30 | 70 | 30 |
| 50 | 30 | 70 |
| 60 | 30 | 70 |
| 60.1 | 90 | 10 |
| 70 | 90 | 10 |
Residual solvent
( 1 ) Take about 1.0 g of this product, accurately weighed, placed in a 10ml volumetric flask, add the diluent ( Dimethyl sulfoxide ) to dissolve, and dilute to the mark. Shake well as the test solution; accurately weigh appropriate amount of Methanol 300mg, Acetic acid 500mg, Acetonitrile 41mg, Ethyl Acetate 500mg, Dichloromethane 60mg, Tert-butyl methyl ether 500mg, Isopropanol 500mg, place them in a 50 ml measuring bottle, add an appropriate amount of diluent to dissolve and dilute to the mark, shake well, take 1 ml of precise amount, place it in a 20 ml measuring bottle, and add the diluent. Dilute to the mark, shake well, as the reference solution. According to the residual solvent determination method, the capillary column with 6% cyanopropylbenzene-94% dimethyl polysiloxane (or similar polar) as the fixing solution is the chromatography column (Agilent DB-624); the initial temperature is 40 ℃, maintained for 15 minutes, heated to 220 ℃ at a rate of 10 ℃per minute, maintained for 2 minutes; inlet temperature is 200 ℃; FID detector temperature is 250 ℃. Flow rate is 3.0ml/min; Split ratio is 5:1; Carrier gas is Nitrogen. Take 1 µl of the reference substance solution and inject it into the gas chromatograph. The resolution between the peaks of each component should meet the requirements. Accurately measure 1µl of the reference solution and the test solution into the gas chromatograph, and record the chromatogram. Calculated by peak area according to the external standard method, it should not exceed 0.3% for methanol, 0.5% for acetic acid, 0.041% for acetonitrile, and 0.5% for ethyl acetate, and 0.06% for dichloromethane, 0.5% for tert-butyl methyl ether, and 0.5% for isopropanol.
( 2 ) Take about 1.0 g of this product, accurately weighed, placed in a 10ml volumetric flask, add the diluent ( N,N-Dimethylformamide ) to dissolve, and dilute to the mark. Shake well as the test solution; accurately weigh appropriate amount of triethylamine 500mg, place it in a 50 ml measuring bottle, add an appropriate amount of diluent to dissolve and dilute to the mark, shake well, take 1 ml of precise amount, place it in a 20 ml measuring bottle, and add the diluent. Dilute to the mark, shake well, as the reference solution. According to the residual solvent determination method, the capillary column with Amine inerts as the fixing solution is the chromatography column ( InertCap for Amines ); the initial temperature is 50 ℃, maintained for 8 minutes, heated to 220 ℃ at a rate of 10 ℃ per minute, maintained for 2 minutes; inlet temperature is 200 ℃; FID detector temperature is 250 ℃. Flow rate is 3.0ml/min; Split ratio is 10:1; Carrier gas is Nitrogen. Accurately measure 1µl of the reference solution and the test solution into the gas chromatograph, and record the chromatogram. Calculated by peak area according to the external standard method, it should not exceed 0.5% for triethylamine.
Assay
According to high performance liquid chromatography (Chinese Pharmacopoeia 2020 Edition General Provisions 0512).
Chromatographic conditions and system suitability tests used octadecylsilane-bonded silica gel as a filler; 0.1% phosphoric acid aqueous solution (5mol/L sodium hydroxide solution adjust the pH value to 3.0) was used as the mobile phase A, and acetonitrile was used as the mobile phase B; Linear elution was performed according to the isocratic elution procedure (mobile phase A – mobile phase B ( 85:15 ) ). The flow rate is 1.0ml per minute; the detection wavelength is 230nm; the column temperature is 30 ℃; Injection volume is 10µl and the time is 20min.
Determination method
Take about 10 mg of this product, accurately weigh it, place it in a 10 ml measuring bottle, add an appropriate amount of solvent (10% acetonitrile) to dissolve and dilute to the mark, shake well, take 1 ml of precise amount, place it in a 20 ml measuring bottle, and add the solvent ( 10% acetonitrile) Dilute to the mark, shake well, as the test product solution, accurately take 10μl and inject it into the liquid chromatograph, record the chromatogram; another take the appropriate amount of the Molnupiravir reference substance and determine by the same method. Calculated by the peak area according to the external standard method.
How to Test Molnupiravir Raw Material (Method of Analysis)

