The SOP for Cleaning Validation and Solubility of Active Ingredients how to approval will be performed after Type A cleaning. After good Cleaning review just, the gear will took into Cleaning validation test. Swab tests and Rinse tests will be gathered to check the presence of dynamic buildup content and Microbiological bio trouble according to given examining plan.
PURPOSE
This procedure describes the strategy and basic requirement for validation of cleaning procedures before the manufacture of drug products which are necessary to prevent chemical and microbiological contamination Cleaning Validation Procedure.
SCOPE
It applies to all sections including Eye drops I.V solutions & SDF. All cleaning procedures which are applied to a specific process / product /system /equipment must be described & evaluated for Cleaning Validation Procedure – Pharma About requirement.
DEFINITION
- E.D Eye Drops
- I.V Intravenous Infusion
- SDF Solid Dosage Form
- OOS Out of Specifications
- QA Quality Assurance
- API Active Product Ingredient
PROCESS & RESPONSIBILITY
- A risk assessment is conducted by a trained person before validation of each cleaning procedure. The Cleaning Validation Procedure – Pharma About following parameters may considered in the risk assessment
- Solubility of APIs and excipients (Annex I).
- Dosage level / toxicity / potency.
- Equipment design and construction (including direct / indirect surface contact area to product).
- Dedicated / Non dedicated Equipment.
- Critical equipment parts.
- Non Sterile / Sterile special consideration of microbiological endotoxin & particulate risks.
- Batch size and possible change over.
- SOP for Cleaning Validation and Solubility of Active Ingredients
- Processing time scales.
- Cleaning intervals
- Idle time and idle conditions.
Following the risk assessment, nitrify worst case situations before writing cleaning validation protocol. Cleaning procedures for equipment’s in which similar products or processes are handled, may not need to be individually validated.
If new equipment is brought to site /building, re-evaluate the assessment for cleaning of the products to be processed in the equipment. If the new equipment is identical in design, construction and material to the existing equipment used for the processes, a revalidation is not required.
Perform three separate pre determined runs of the cleaning procedure, their success prove that the method is validated. For cleaning validation establish & approve the following documents in the following order:
Validation Master Plan.
Include a cleaning validation of particular equipment in Validation Master Plan as prepared on yearly basis with the cooperation of concerned department.
Cleaning Validation Protocol.
- Issue the validation protocol authorized by production & QA prior to conducting the validation study.
- The protocol describes in detail how the validation of a specific cleaning procedure will be performed, what the equipment and drug products concerned are and who is responsible for execution, review, approval and documentation.
- The cleaning validation protocol covers the following topics:
- SOP for Cleaning Validation and Solubility of Active Ingredients
- i. Unit Operation
- ii. Purpose
- iii. Scope
- iv. Responsibility
- v. Acceptance criteria
- vi. Sampling Procedure (Direct surface sampling or Swab, Use of rinse solution)
- vii. Testing Procedure (Analytical, Microbiological)
- viii. Cleaning validation report (Analytical & Microbiological).
Cleaning validation report
- A cleaning validation report documents the validation activities performed. Validation activities are considered functionally complete when all raw data have been generated, reviewed & found acceptable. The results of all tests are summarized for all contaminants
- Review all deviations and changes that occurred during the validation. If the key acceptance criteria have failed, investigate in order to determine possible laboratory errors (e.g. analytical equipment, analytical method and sampling) as per SOP/QA/00. (Investigation of OOS results) (SOR/QA/00-00)
- Review all results with regard to acceptance criteria and compare with expected values. For any unexpected deviations of cleaning parameters or analytical OOS results, discuss the effects on the product quality and draw appropriate conclusions.
- QA and production is authorized for the acceptance / Rejection decision after the completion of all activities including any corrective actions and repetitions in validation report.
Revalidation may be necessary, if a new worst case is introduced or the cleaning procedure is changed. Changes can influence the validation status, to control these changes follow change control procedure (SOP/QA/54).
In case of a new drug product induction to a site / building, re-evaluate the risk assessment for cleaning of the equipment used for this product. If the new product is not covered by existing cleaning validation, perform a revalidation with this drug product.
RECORD SOP for Cleaning Validation and Solubility of Active Ingredients
Solubility of Active Ingredients Annex-I
REFRENCE
- Change Control Procedure
- Validation Master Plan
- Investigation of OOS Results
- Request for analysis
DISTRIBUTION SOP for Cleaning Validation and Solubility of Active Ingredients
- HOD QA/QC
- Plant Manager
- All concerned staff
- AMENDMENTS
- A-First Issue
- B – Amendments are made in 4.1, 4.4.3.2 to incorporate ref. document.
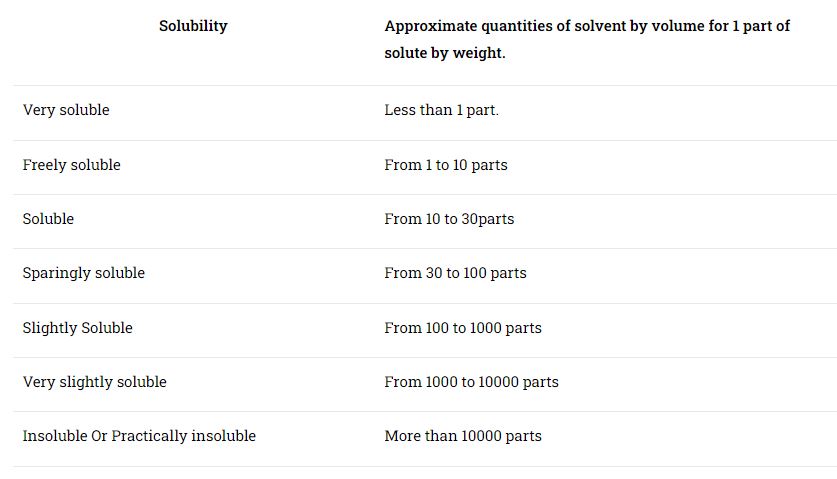
Solubility of Active Ingredients
- Chloramphenicol Slightly soluble in water, freely soluble in alcohol and in propylene glycol.
- Dexamethasone sod. phosphate Soluble in water, sparingly soluble in ethanol, slightly soluble in CH2 Cl2
- Antazoline HCl Sparingly soluble in water, soluble in alcohol, slightly soluble in methylene chloride.
- Diclofenac Sodium Sparingly soluble in water, freely soluble in methanol soluble in alcohol, slightly soluble in acetone, in soluble in ether
- Sorbic acid Slightly soluble in water, freely soluble in alcohol.
- di-sodium edetate Soluble in water, sparingly soluble in alcohol, in soluble in ether
- Fluorometholone In soluble in water, slightly soluble in absolute ethanol and ether
- α-tocopherol acetate Freely miscible with ethanol, acetone and ether, produce a stable opalescent emulsion with H2O
- Tetrahydrozolien HCl Freely soluble in water and alcohol very slightly soluble in chloroform Practically Insoluble in ether
- Timolol Maleate Soluble in water and in alcohol, practically insoluble in ether
- Potassium iodide Very soluble in water, freely soluble in glycerol and soluble in alcohol
- Sodium iodide Very soluble in water, freely soluble in alcohol.
- Adenosine Slightly soluble in water, soluble in hot water, practically insoluble in ethanol (96 per cent) and in methylene chloride. It dissolves in dilute mineral acids.
- Anhydrous sod. succinate Soluble in water, partially soluble in alcohol.
- Atorvastatin Calcium Soluble in water.
- Azithromycin dihydrate Practically insoluble in water, freely soluble in anhydrous ethanol and in methylene chloride.
- Benzalkonium chloride Very soluble in water and in alcohol.
- Calcium chloride White or almost white, crystalline powder, hygroscopic.
- Carvedilol EP Soluble in water.
- Ciprofloxacin HCl Soluble in water, slightly soluble in methanol, very slightly soluble in ethanol, practically Insoluble in acetone, in ethyl acetate and in methylene chloride.
- Citric acid anhydrous Very soluble in water, freely soluble in ethanol (96 per cent).
- Clopidogrel bisulphate Soluble in ethanol and insoluble in ether.
- Cytidine Practically insoluble in water and in alcohol.
- Cytochrome-C Soluble in water & insoluble in alcohol.
- Dexamethasone base Practically insoluble in water, sparingly soluble in anhydrous ethanol, slightly soluble in methylene chloride.
- Dextrose anhydrous Freely soluble in water, sparingly soluble in ethanol (96 per cent).
- Dextrose monohydrate Freely soluble in water, sparingly soluble in ethanol (96 per cent).
- Erythromycin stearate Practically insoluble in water, soluble in acetone and in methanol. Solutions may be opalescent.
- Fructose Very soluble in water, soluble in ethanol (96 per cent).
- Gaunosine-5-Di-sodium phosphate Soluble in water, insoluble in alcohol & ether.
- Getamycin sulphate Freely soluble in water, practically insoluble in alcohol.
- Glimepride Practically insoluble in water, soluble in dimethylformamide, slightly soluble in methylene chloride, very slightly soluble in methanol.
- Glycine Freely soluble in water, very slightly soluble in ethanol (96 per cent).
- Hydrochlorothiazide Very slightly soluble in water, soluble in acetone, sparingly soluble in ethanol (96 per cent).
- Hydroxyethyl starch Soluble in hot and cold water giving a colloidal solution, practically insoluble in acetone, in ethanol (96 per cent) and in toluene.
- Hydroxypropyl methyl cellulose Soluble in cold water, in glacial acetic acid, in anhydrous ethanol, in methanol and in propylene glycol and in a mixture of 10 parts of methanol.
- Levofloxacin Slightly soluble in water, soluble in 0.1N HCl.
- Losartan potassium Freely soluble in water slightly soluble in alcohol.
- Loratadine Practically insoluble in water, freely soluble in acetone and in methanol.
- Magnesium chloride 6H2O Very soluble in water, freely soluble in ethanol (96 per cent).
- Magnesium stearate Practically insoluble in water and in ethanol.
- Mannitol Soluble in water and 0.1N HCl
- Metronidazole Freely soluble in water, very slightly soluble in ethanol (96 per cent).
- Methocil (HPMC) Soluble in water and 0.1N HCl
- Moxifloxacin Practically insoluble in hot water, in acetone, in anhydrous ethanol and in toluene.
- Naaga sodium salt Soluble in water completely.
- Naphazoline nitrate Sparingly soluble in water, soluble in ethanol (96 per cent).
- Neomycin sulphate Very soluble in water, very slightly soluble in alcohol, practically insoluble in acetone.
- Nicotinamide Freely soluble in water and in ethanol.
- Omeprazole pallets Soluble in 0.1N NaOH and in methanol.
- Ofloxacin Very slightly soluble in water, soluble in methylene chloride, sparingly soluble in ethanol (96per cent) and in methanol.
- Paracetamol Sparingly soluble in water, freely soluble in alcohol, very slightly soluble in methylene chloride.
- Phenyl mercuric nitrate Very slightly soluble in water and in ethanol (96 per cent), slightly soluble in hot water.
- Phenylephrine HCl Freely soluble in water and in ethanol (96 per cent).
- Pilocarpine nitrate Freely soluble in water sparingly soluble in alcohol.
- Polymixin B. sulphate Soluble in water, slightly soluble in ethanol (96 per cent).
- Polyoxyl 35 Freely soluble in water & in alcohol.
- Potassium chloride Freely soluble in water, practically insoluble in anhydrous ethanol.
- Pseudoephdrine HCl Freely soluble in water and in ethanol (96 per cent), sparingly soluble in methylene chloride.
- Sodium acetate anhydrous Very soluble in water, soluble in ethanol (96 per cent).
- Sodium acetate 3H2O Very soluble in water, soluble in ethanol (96 per cent).
- Sodium bicarbonate Soluble in water, practically insoluble in ethanol (96 per cent).
- Sodium chloride Freely soluble in water, practically insoluble in anhydrous ethanol.
- Sodium citrate Freely soluble in water, practically insoluble in ethanol (96 per cent).
- Sodium lactate Miscible with water and with alcohol.
- Sorbitol Very soluble in water, practically insoluble in ethanol (96 per cent).
- Sulphacetamide Freely soluble in water, slightly soluble in anhydrous ethanol.
- Thimerosal Freely soluble in water, sparingly soluble or soluble in ethanol (96 per cent), practically insoluble in methylene chloride.
- Thymidine Freely soluble in water.
- Tobramycin sulphate Completely soluble in water, slightly soluble in alcohol.
- Tropicamide Slightly soluble in water, freely soluble in alcohol and in methylene chloride.
- Uridine Soluble in water, very slightly soluble in alcohol.

