The purpose of this SOP for Corrective and Preventive Action CAPA is to establish a systematic and standardized procedure for identifying, implementing, and documenting Corrective and Preventive Actions (CAPA) to address non-conformities, prevent their recurrence, and continually improve processes within pharmaegg.com.
PURPOSE of SOP for Corrective and Preventive Action CAPA
- The purpose of the corrective and preventive action (CAPA) is to collect information, analyze information, identify and investigate product and quality problems, and take appropriate and effective corrective and/or preventive action to prevent their recurrence. Verifying or validating corrective and preventive actions, communicating corrective and preventive action activities to responsible people, providing relevant information for management review, and documenting these activities are essential in dealing effectively with product and quality problems, preventing their recurrence, and preventing or minimizing device failures.
- To provide a sequence of steps to identify, assess, evaluate, implement and
monitor solution addressing actual and potential non-conformities, defects or other.
undesirable situations in order to prevent recurrence. - Reduction in recalls & incidents caused by known problems and issues.
- Reduction in adverse inspection observations.
- More robust and consistent production processes and increased customer satisfaction.
- Mitigation of risk where it is not possible to eliminate the root cause.
- To periodically review the quality system for its continuing suitability and effectiveness.
SCOPE
- This SOP covers the requirements for CAPA Process for incident/ deviation having significant risk to patient safety, product quality, compliance or pose a significant risk to the business/ process efficiency (e.g. due to recurrence), it may be trigger from the following areas (as minimum):
• Incidents and Recalls
• Regulatory Inspections
• External Audits
• Quality Audits
• Self-Inspections
• Annual Product Review
• Deviation Handling Reports
• Out of Specification Results Investigations
• Risk Management - This document complies with WHO and PNAC (Pakistan National Accreditation Council) ISO/IEC 23025:2023(E) standard.
ROLES & RESPONSIBILITIES
| Role | Responsibilities |
| Departmental Managers/Designee/QA officer |
|
| CAPA Team/Management |
|
| QA Department |
|
| QA Manager |
|
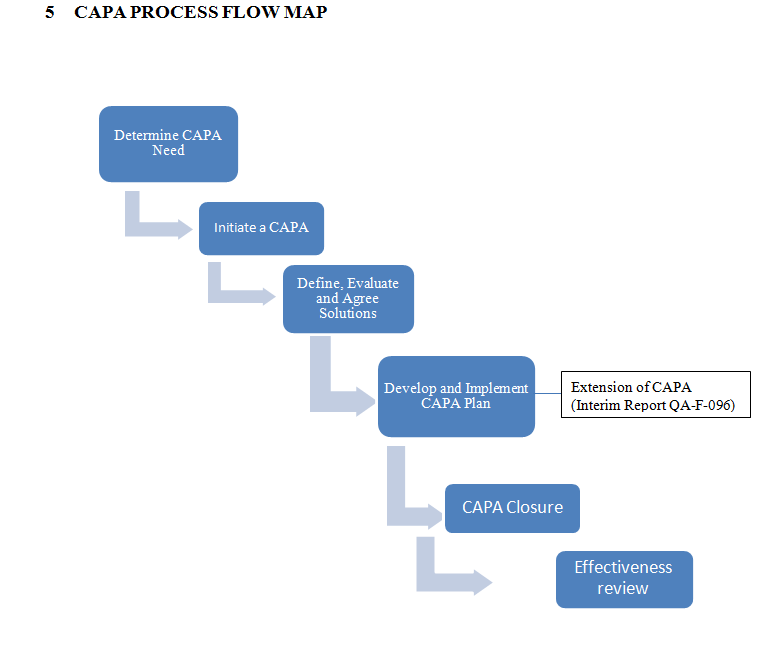
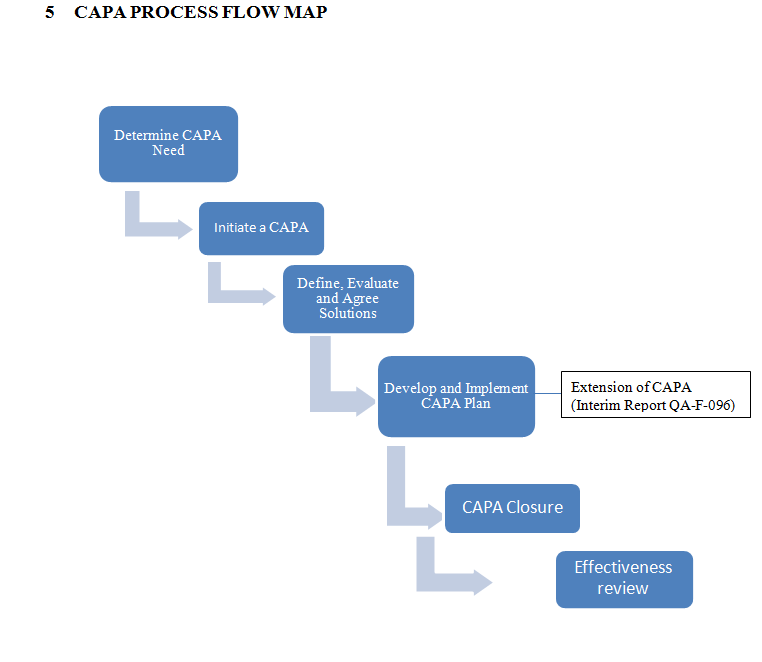
PROCEDURE
Determine the Need to apply CAPA Process
- When information is received or gathered from an incident/ issue, an assessment review will be performed by the Quality Forum as to whether a documented CAPA should be raised or not.
- Based on available data a decision is made as to whether the outcome is a ‘just do it’ situation or there is a need to initiate CAPA process.
- The need to raise the CAPA is considered in the event of repeated occurrences of the same or similar non-compliances & criticality e.g. where there is indication of inefficient processes /inadequate remedial actions & recurrence in future that pose a significant risk to patient safety, product quality, compliance& business/ process efficiency.
Initiate CAPA
Assign a CAPA Leader
- Concerned Department Manager/ Designee will lead the CAPA process.
- The CAPA leader will determine whether a CAPA Team is required, if so, the team members will be selected. The name of CAPA team will be documented.
- The need and size of CAPA team will depend on the nature of the CAPA objective e.g. 01 person can be owner.
- The CAPA Leader and CAPA team must be skilled and experienced.
- SOP for Corrective and Preventive Action CAPA
Determine the Root Cause and Prepare CAPA Objective
- The CAPA team will determine or confirm the root cause, associated risks & scope of the CAPA.
- The CAPA team will prepare CAPA Objective on the basis of available data.
- Consideration must be given to the criticality/priority in defining the CAPA objective to ensure the CAPA is progressed in a timely manner.
Define, Evaluate and Agree Solutions
- If a direct link between the root cause and incident/issue is established, an effective corrective and preventive action is readily evident, a single CAPA solution is acceptable. If a single CAPA solution is not readily evident then the CAPA Team will identify all the possible solution options to address the root cause.
- Brainstorming method can be used to identify the solution options.
- Where it is not possible to eliminate the root cause then assess the associated risks and mitigate it by preparing a mitigation plan including suitable solution options.
- Based on the assessment, the CAPA team will select the best solution option and record the decision.
- CAPA leader must assess the requirement of change control.
- The agreed solution & the requirement of change Control must be approved by Quality Assurance Manager.
Develop and Implement SOP for Corrective and Preventive Action CAPA
- The CAPA leader will develop a plan for the agreed solution, clearly identifying owners for each action.
- The CAPA leader with Quality Assurance Manager will approve the CAPA Plan.
- The CAPA leader must ensure the CAPA plan is in place, implemented and progress against the developed Plan until completion.
- The CAPA leader must collect the necessary documents as required in the CAPA plan.
- Any significant risk arising from deviation, changes or delays to the plan must be agreed by the Quality Forum, which must be documented by the CAPA leader.
- The CAPA leader will communicate to Quality Assurance Manager on the completion of CAPA plan. The effectiveness of the implemented CAPA will be assessed by CAPA leader and Quality Assurance Manager and documenting a post implementation review.
CAPA Closure
- The CAPA Leader will record the closure of the CAPA after post implementation review.
- CAPA Leader must ensure that all documentation is complete & included with the CAPA document.
- Approval of the QA Manager will be taken at this stage.
- When a solution is adopted into routine use, the CAPA leader must ensure ownership of any on-going effectiveness measures is transferred, if required, to the relevant department
- Once the implemented CAPA is closed, any interim control must be removed.
Assigning number to CAPA
Quality Assurance Manager will allocate the number. Each CAPA will have alphanumeric code as below:
CAPA No. 01/MM-2023
Where,
CAPA stands for Corrective and Preventive Action
01 representing the sequential number.
MM representing the month.
2023 representing the year.
NOTE: Numbering sequence for Audit’s CAPA will be the Audit finding ID or number. SOP for Corrective and Preventive Action CAPA
Document Filing
Original CAPA form will be available from Quality Assurance department and a copy of the completed CAPA form will be with the concerned department.
Interim Report:
- Interim records are required for CAPA’s if the due date for QA decision cannot be adhered to target date.
- In case of extension, concerned client must be informed.
- Interim report must be approved by QA and CEO.
Interim reports must include the following information:
- Current status of the CAPA including action taken in the past 30 calendar days
- Justification / rationale for extending the CAPA an additional 30 calendar days.
- Ongoing and planned actions, including timelines and responsibilities for the actions.
- Responsibility for follow up-monitoring and ongoing reporting and notification.
- Estimated timeline to finalize and closure of CAPA.
GLOSSARY
CAPA = Corrective and preventive action
ID = Identification
QA = Quality Assurance
CHANGE HISTORY
| DATE | Supersede SOP number | CLAUSE# | CHANGE MADE |
| QAG-WI-000 Revision No.05 | — | SOP pattern changed as per SOP for SOPs (QA-G-GRM-000) | |
| Heading 3 | Role and responsibilities incorporated | ||
| Heading 6 | Glossary incorporated | ||
| 8.2 | CAPA tracker form incorporated. | ||
| 8.3 | Effectives review form incorporated. | ||
| Heading 9 | Reference incorporated | ||
| Heading 10 | Distribution List incorporated | ||
| QA-G-CAPA-11 Revision No.06 | All Headers | Exclusion of CEO signatures from Approval | |
| Point No.4 | Flow Chart Revised | ||
| Point 8.4 | Interim Report of CAPA incorporated. | ||
| Point No.5.6 | Month incorporated in CAPA no. |
RELATED DOCUMENTS
- CAPA form QA-F-000
- CAPA tracker QA-F-000
- Effectiveness Review QA-F-000
- Interim Report of CAPA QA-F-000
REFERENCE
- ICH Guidelines Q10, Corrective Action and Preventive Action system, Point # 3.2.2, 3.2.4
- WHO_TRS_908
- ISO/IEC 23025:2013(E) 8.7 Corrective actions
DISTRIBUTION LIST
- Production
- Quality Control
- Engineering
- Warehouse

