
A Standard Operating Procedure SOP for Stability Studies in the pharmaceutical industry outlines the step-by-step procedures and guidelines for conducting stability testing on drug products. Stability studies are essential to assess the long-term stability, efficacy, and quality of pharmaceutical products under various environmental conditions. Below is a general outline for an SOP for Stability Studies
PURPOSE
The purpose of this SOP is to describe the procedure for sample collection, selection of batches, incubation, withdrawal, analysis, reporting, and evaluation, discontinuation, and documentation of stability studies of the drug products.
SCOPE of SOP for Stability Studies
This SOP is applicable for carrying out stability studies of Finished products manufactured at www.pharmaegg.com Group of companies.
ROLES & RESPONSIBILITIES
| Roles | Responsibilities |
| Documentation Incharge |
|
| QA Executive |
|
| QC Analyst |
|
| QC Manager/Designee |
|
| QA Manger |
|
PROCEDURE
General Instructions for stability study:
- Incubate the amount of sample specified in the respective protocol including quantity for analysis as well as for investigation purpose in case of OOT/OOS results.
- Label properly for all the samples incubated should be properly labeled with the condition, orientation and stability study intervals for traceability, and to facilitate the reconciliation while withdrawing a sample from the incubator.
- Prepare the stability study protocol as per SOP for Stability Studies
- Withdraw the stability study sample and analyzed as per the stipulated time window/ due date defined in the protocol.
- Investigate any failure (OOS/OOT) observed during stability study and documented as per the investigation procedure specified in the SOP of OOS and OOT investigation respectively.
- Whenever any batch is found failing in stability study after expiry interval, then there is no need to perform analysis of further intervals.
- For such products, Stability Study Protocol is to be revised and future stability study batches are to be monitored only up to expiry interval or up to the interval till the product is stable whichever is later.
- The stability study followed, the number of batches incubated, the reason for monitoring, condition of stability incubator/walk-in chamber, any excursion or deviation of stability result trend, stability failure, etc. but not limited to, should be evaluated and appropriate action if required should be initiated.
- Conduct stability study testing on the dosage form packaged in the container closure system proposed for marketing (including, as appropriate, any secondary packaging and container label).
- Consider the first three batches for long term and Accelerated stability study. (“First” means the product is manufactured for the first time at the location).
- Batches should be of the same formulation and packaged in the same container closure system as proposed for marketing.
- In the case of a new product, samples of three consecutive batches shall be kept for Accelerated and long-term stability studies.
- Three consecutive batches mean, three batches of each strength of product X manufactured, which can have batch numbers ABC0001, ABC0005 & ABC0007, where, in-between batch numbers can be of any other product. In case of specific requirements, QA shall provide the samples to QC for stability study analysis accordingly.
Storage condition of Stability Study Samples:
Refer Table-1 for different temperature and humidity conditions for the long term, Accelerated and intermediate condition as follows.
Table 1
| Stability Conditions (For Accelerated) | Stability Conditions (For Long Term) | ||
| Temperature | Humidity | Temperature | Humidity |
| 40oC ±2oC | 75% Rh ±5% | 30oC ±2oC | 65% Rh ±5% |
Temperature / RH Monitoring of Stability Chambers:
- Temperature / RH of stability chamber shall be recorded on the basis of 4 hour.
- Temperature / RH shall be monitored through software on the interval of 1 hour and print out shall be taken on daily basis. This printout shall be reviewed by Executive stability / Nominee.
- If any failure in Temperature / RH of Stability Chambers found, investigation shall be done and note down in the Stability chamber failure investigation report and CAPA shall be generated as per respective SOP.
Stability Specification SOP for Stability Studies
- The testing requirements shall be defined in the Stability Study Protocol and shall cover as appropriate, the physical, chemical, microbiological preservative, and functionality tests.
- Stability acceptance criteria should be derived from the consideration of all available stability study information.
- It may be appropriate to have the justifiable difference between the Stability and release acceptance criteria based on the stability study evaluation and the changes observed on storage.
- In the case of the pharmacopoeia drug products, criteria of release and stability specification will be as per monograph.
- In such a case tighter internal release limit needs to be retained to get drug products within the criteria during the shelf life.
- Whenever specification is updated for any reason (like pharmacopoeial change but not limited to) and causes a change in analytical method, acceptance criteria, test inclusion/deletion, etc. update the associated Stability Study Protocol which shows the required changes e.g. specification No., acceptance criteria, etc.
- If, after the change of analytical method immediate analyzed stability study station samples fall under the criteria of OOT or OOS then investigation shall be performed using the previous analytical method before concluding that it’s a stability study impact or a method impact on the drug product.
Stability Study Protocol
- QA shall submit a request with all relevant information required for protocol preparation for the quality control department.
- Stability Coordinator/designee of the quality control department shall prepare the Stability Protocol (based on requirement and project).
- Prepare the stability protocol for the different requirements e.g. different packs, customer and regulatory agency, etc.
- QA shall review and approve the protocol.
- Issue the stability study protocol prior to the execution of the stability study.
- The sample quantity charged per station shall be 1.5 times of the actual required quantity for one-time analysis /per batch /per station/ storage condition. If not then it should be justified.
- Additional sample quantity shall be incubated/date in for accelerated & intermediate stability study conditions, equivalent to two SOP for Stability Studies station quantities for chemical analysis & one stability station quantity for microbiology analysis.
- For Long Term condition, three stability station sample quantity for chemical analysis and one stability station sample quantity for microbiological analysis should be kept performing any analysis OOT, OOS, Regulatory deficiency analysis, and retesting because of analytical method revision, etc.
- The change control procedure shall be followed if the protocol to be revised based on the requirement.
- QA shall refer to or confirm the stability sample quantity from the stability study protocol.
Initiation of stability
The quality assurance shall select the batches for stability studies.
| Category | Stability storage condition | Selections of batches |
| New drug product | Long term, ACC & Intermediate (if applicable) | First Three batches |
| Annual addition batch | Long term | One batch |
| Changes in the primary packaging/closure material/system (lining, rubber, type of sealing, cap and sterilization process) | Long term & ACC
SOP for Stability Studies |
One batch Note: Three batches shall be charged if the change in the MOC of primary packaging (evaluation of selecting the No. of batches shall depend upon the type of changes) |
| API source change | Long term ACC | One batch |
| Simulated bulk transfer pack | Long term | As per respective protocol |
Withdrawal of Stability Samples from Incubators:
- Withdraw stability samples (for all the conditions) from the stability chambers as on due date of withdrawal.
- In case if required withdrawal shall not be extended for +3 days (calendar) from the due date. The Stability Coordinator or designee shall make entries of the sample withdrawn in the “Stability Sample Reconciliation log book Form No. 000
- The stability coordinator or designee shall withdraw the required sample quantity as specified in the Stability Study Protocol.
- Record the details of the sample quantity withdrawn in the reconciliation Logbook form no. QA-F-00
- Store the samples withdrawn for stability study under control room temperature conditions until the analysis and review.
Note: Products which require some specific Temperature and relative humidity condition or highly sensitive to changing environmental condition shall be taken care as per the label claim or recommendation conditions and shall be stored in respective label claim condition.
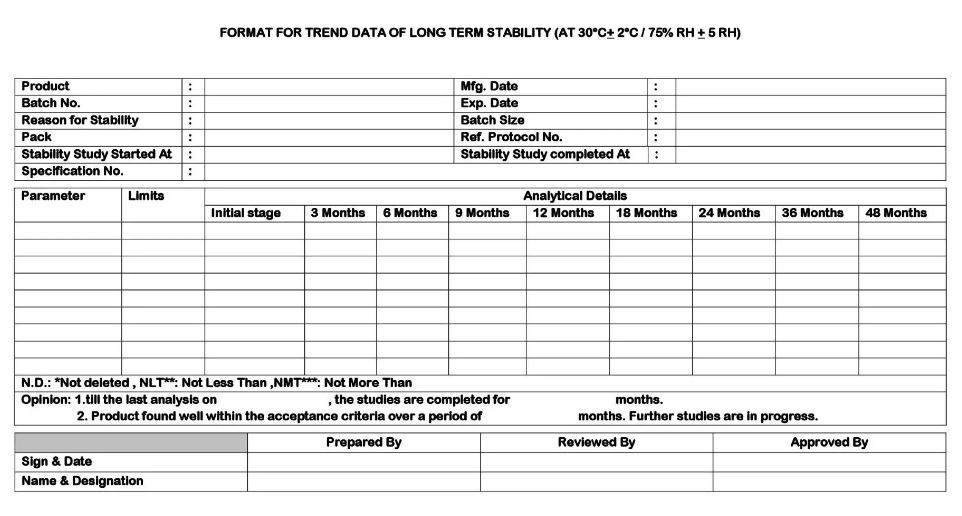
Reporting of Stability Study results:
- The analyst shall analyze the samples as per the test procedures given in the stability study protocol. On completion of the analysis of all the tests, the analyst shall enter the results in the Stability study summary report.
- However, the stability study summary report can be modified with respect to more information based on customer requirements.
- The analyst shall attach the chromatograms, UV spectrum, etc. with the template and shall submit the analytical along with the raw data for review to the designed person.
- After approval of the report, Stability study Summary Report of the respective product /batch shall be updated.
- Any result, which is found out of specification or Out of trend, shall be intimated to the QA Manager immediately.
- QA Manager along with QC Manager shall investigate the Out of Specification (OOS) results according to the SOP “Investigation of Out of Specification Analysis Result”.
- Take the joint decision for Recall / Revision of formulation.
- On completion of the stability study schedule of the product batch, the Quality Control Manager or designee shall give final conclusion and the report shall be submitted to the QA Manager for the approval.
- On receipt of the approval, enter the data in the stability study protocol cum report (Summary Report) and file the documents in a stability file.
REFERENCE
ICH SOP for Stability Studies

