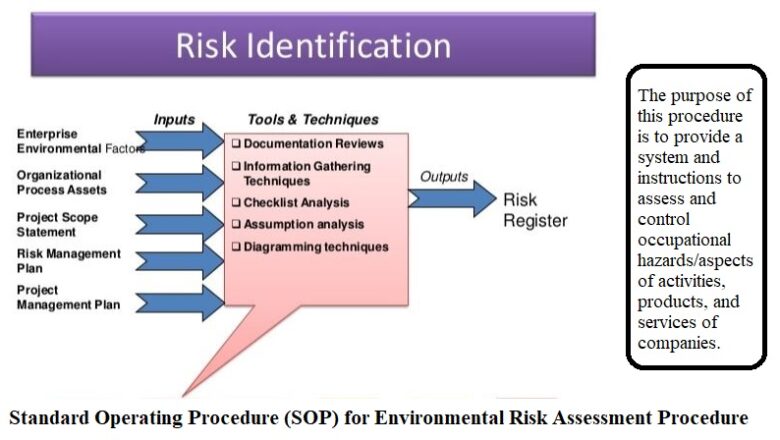
Determining Shelf Life And Stability Testing Programme
Standard Operating Procedure for Determining Shelf Life And Stability Testing Programme
Determining Shelf Life And Stability Testing Programme is the stability testing programmed for all pharmaceutical products manufactured at Company facility confirm the stability of the products and compatibility.
PURPOSE
To describe the stability testing programmed for all pharmaceutical products manufactured at Company facility.
To confirm the stability of the products and compatibility with its container, while stored in accordance with labeling conditions and time, and with respect to product degradation. Pharmaceutical products will be tested at designated intervals for certain physical, chemical and microbiological properties.
To collect scientific evidence based on test results to d...