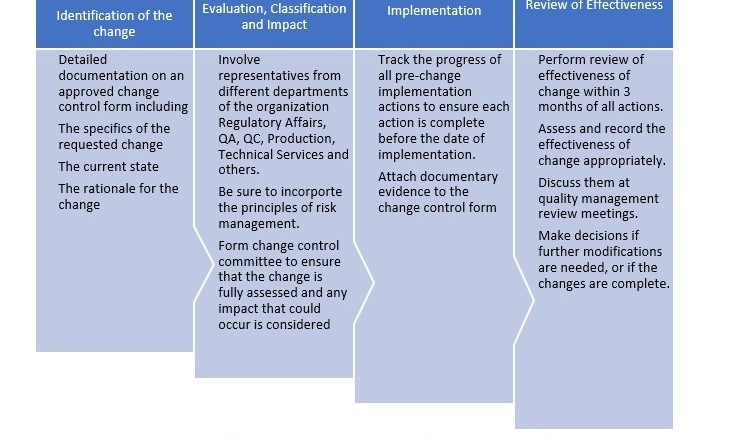
SOP for Amlodipine 5mg/Valsartan 160mg/Hydrochlorothiazide 12.5mg Tablet Assay Testing
It is important to follow appropriate safety precautions when handling these drugs and to adhere to the manufacturer's instructions for the assay procedure of SOP for Amlodipine 5mg/Valsartan 160mg/Hydrochlorothiazide 12.5mg Tablet Assay Testing.
PURPOSE:
To describe the procedure for analysis at the in-process and finished stage of SOP for Amlodipine 5mg/Valsartan 160mg/Hydrochlorothiazide 12.5mg Tablet Assay Testing.
SCOPE:
This SAP gives a detailed outline for the finished product analysis of SOP for Amlodipine 5mg/Valsartan 160mg/Hydrochlorothiazide 12.5mg Tablet Assay Testing and will cover In process testing activities on physical, chemical & instrumental basis.
RESPONSIBILITY:
QC Analyst is responsible for physical / chemical testing and preparing standard analytical proce...