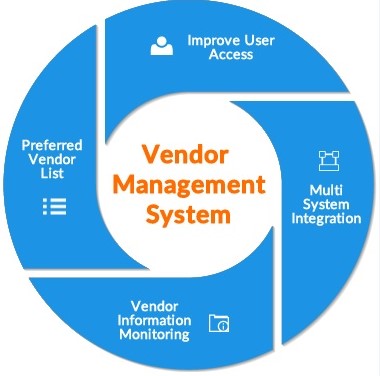
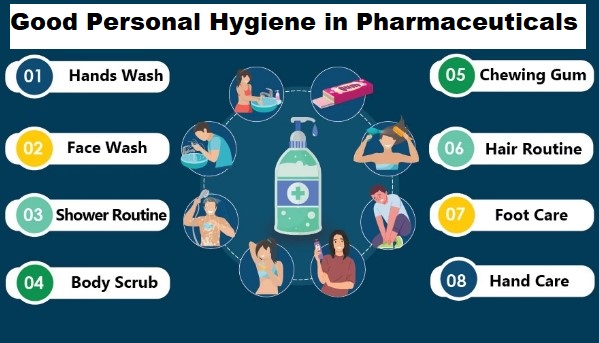
Tablet Making Process in Pharmaceuticals – Step by step guide
The tablet making process in the pharmaceutical industry is a crucial and complex procedure that involves several steps to ensure the final product is of high quality and meets the required standards. From the selection of active ingredients and excipients to inspection and packaging, each step plays an essential role in determining the quality of the final tablet. In this article, we will delve into the complete tablet manufacturing process in pharmaceuticals, providing an in-depth look at each step, from blend preparation to stability testing. With a focus on precision and expertise, this guide will provide a comprehensive overview of the art of tablet making in the pharma industry.
The process of making tablets in the pharmaceutical industry typically involves the following steps:
Ste...