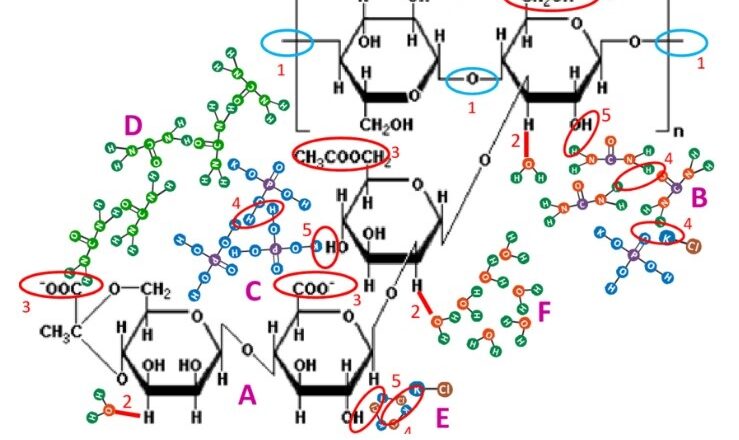
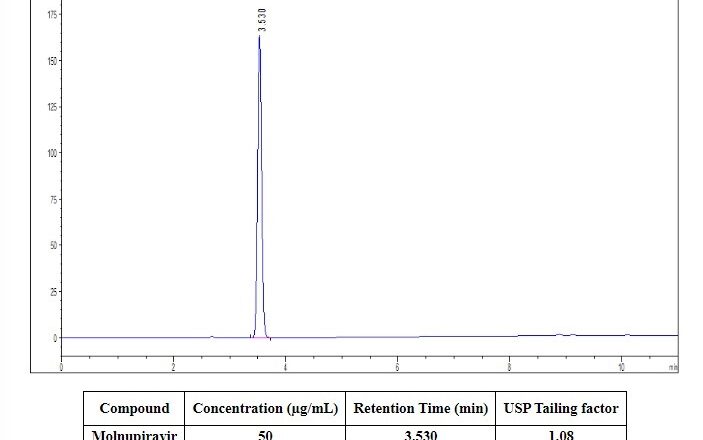
Risk Management in Pharmaceuticals
Risk management in pharmaceuticals is the systematic process of identifying, assessing, and prioritizing potential risks associated with the development, production, and distribution of drugs, and implementing measures to mitigate these risks. This involves identifying and assessing the potential risks associated with each stage of the drug development process, from the initial discovery and development of new compounds to the post-market monitoring of drugs once they are on the market. Risk mitigation strategies may include changes to the drug's formulation, manufacturing processes, labeling, or distribution methods, as well as increased monitoring and clinical testing. The goal of risk management in pharmaceuticals is to ensure that drugs are safe and effective for patients, while also b...