General SOP for Batch Processing
Standard Operating Procedure (SOP) for Review of General SOP for Batch Processing in the pharmaceutical manufacturing industry. A detailed checklist for review of General SOP for Batch Processing for Draft Copy as well as Filled Copy of Batch Manufacturing/Packing Records. this SOP is to define the procedure to review the draft General SOP for Batch Processing prior to final approval. Also, this SOP shall be applicable for a review of executed BMR prior to the final release of batches.
PURPOSE :
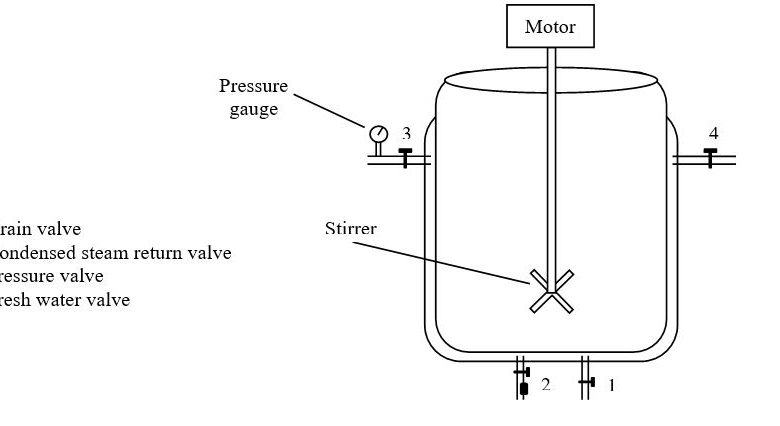
To lay down the procedure for manufacturing Tablets and Capsules.
SCOPE:
This SOP shall be applicable for Tablet & Capsule Manufacturing in the Production department.
RESPONSIBILITY:
1 Execution: Production Pharmacist.
2 Checking: Production Manager.
3 Q.A Officer.
REFERENCES:
In-hous...