SOP for Conduction of Validation Study
A Standard Operating Procedure SOP for Conduction of Validation Study is critical in the pharmaceutical industry to ensure that processes, equipment, and systems are validated according to regulatory requirements.
This procedure SOP for Conduction of Validation Study is to establish a systematic and documented approach for planning, conducting, and documenting validation studies to ensure that processes, equipment, and systems meet predetermined acceptance criteria and comply with regulatory requirements.
Objective:
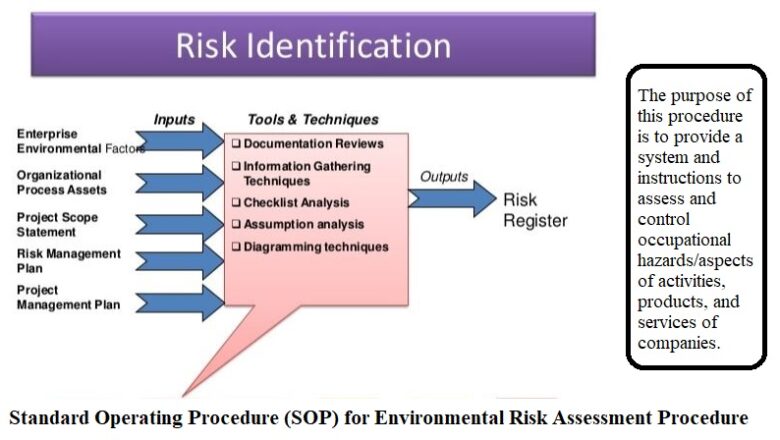
To establish and maintain product integrity, performance and reliability, and to ensure that systems, procedures and equipments are clearly defined, functioning correctly, and are shown to be equipped to achieve the desired results.
SOP for Conduction of Validation Stud...