SOP for Corrective and Preventive Action CAPA
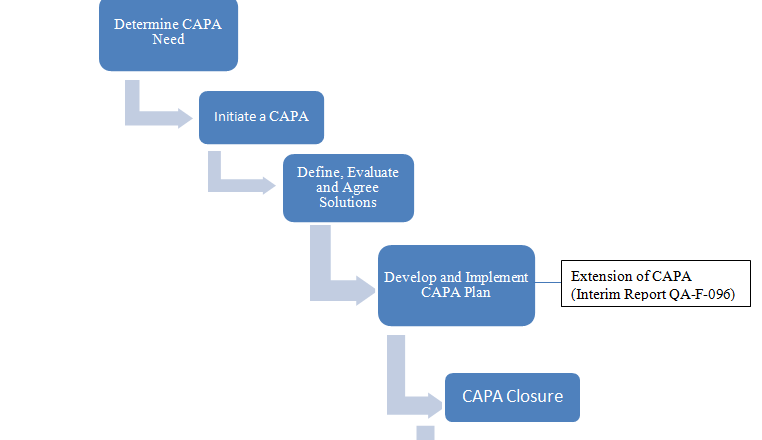
The purpose of this SOP for Corrective and Preventive Action CAPA is to establish a systematic and standardized procedure for identifying, implementing, and documenting Corrective and Preventive Actions (CAPA) to address non-conformities, prevent their recurrence, and continually improve processes within pharmaegg.com.
PURPOSE of SOP for Corrective and Preventive Action CAPA
The purpose of the corrective and preventive action (CAPA) is to collect information, analyze information, identify and investigate product and quality problems, and take appropriate and effective corrective and/or preventive action to prevent their recurrence. Verifying or validating corrective and preventive actions, communicating corrective and preventive action activities to responsible people, providing rele...










