SOP for cGMP Internal Audit / Self Inspection
A Standard Operating Procedure SOP for cGMP Internal Audit / Self Inspection in the pharmaceutical industry is vital for ensuring compliance with Good Manufacturing Practice (cGMP) guidelines and maintaining a high level of quality and safety in the manufacturing process. Below is a general outline for creating an SOP for cGMP Internal Audit or Self-Inspection
Purpose
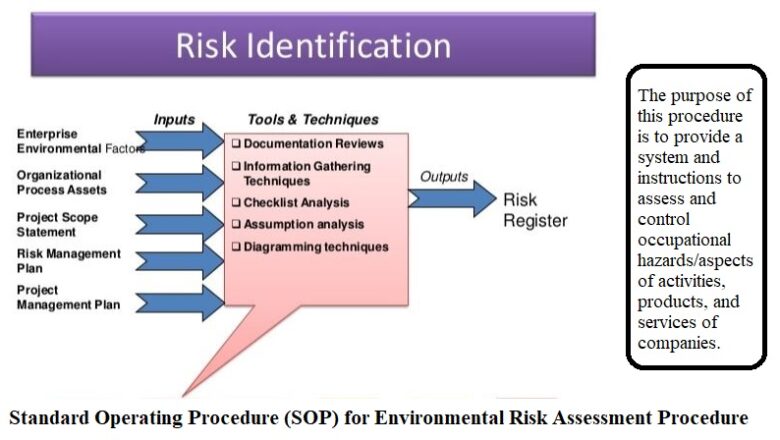
To describe procedure for planning, executing, reporting and follow up of cGMP internal audit as well as Self Inspection
SOP for cGMP Internal Audit / Self Inspection Scope
The scope of this document pertains to all regulated departments including, Production, Quality Control, Quality Assurance, Procurement, Engineering, Warehouses and IT department of Pacific Pharmaceuticals Ltd
Responsibilities
QA Head is respon...